Mass Spectrometry-Based Evaluation of the Bland-Altman Approach: Review, Discussion, and Proposal
- PMID: 37446566
- PMCID: PMC10343610
- DOI: 10.3390/molecules28134905
Mass Spectrometry-Based Evaluation of the Bland-Altman Approach: Review, Discussion, and Proposal
Abstract
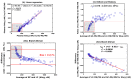
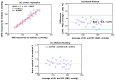
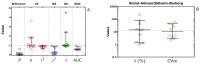
Reliable quantification in biological systems of endogenous low- and high-molecular substances, drugs and their metabolites, is of particular importance in diagnosis and therapy, and in basic and clinical research. The analytical characteristics of analytical approaches have many differences, including in core features such as accuracy, precision, specificity, and limits of detection (LOD) and quantitation (LOQ). Several different mathematic approaches were developed and used for the comparison of two analytical methods applied to the same chemical compound in the same biological sample. Generally, comparisons of results obtained by two analytical methods yields different quantitative results. Yet, which mathematical approach gives the most reliable results? Which mathematical approach is best suited to demonstrate agreement between the methods, or the superiority of an analytical method A over analytical method B? The simplest and most frequently used method of comparison is the linear regression analysis of data observed by method A (y) and the data observed by method B (x): y = α + βx. In 1986, Bland and Altman indicated that linear regression analysis, notably the use of the correlation coefficient, is inappropriate for method-comparison. Instead, Bland and Altman have suggested an alternative approach, which is generally known as the Bland-Altman approach. Originally, this method of comparison was applied in medicine, for instance, to measure blood pressure by two devices. The Bland-Altman approach was rapidly adapted in analytical chemistry and in clinical chemistry. To date, the approach suggested by Bland-Altman approach is one of the most widely used mathematical approaches for method-comparison. With about 37,000 citations, the original paper published in the journal The Lancet in 1986 is among the most frequently cited scientific papers in this area to date. Nevertheless, the Bland-Altman approach has not been really set on a quantitative basis. No criteria have been proposed thus far, in which the Bland-Altman approach can form the basis on which analytical agreement or the better analytical method can be demonstrated. In this article, the Bland-Altman approach is re-valuated from a quantitative bioanalytical perspective, and an attempt is made to propose acceptance criteria. For this purpose, different analytical methods were compared with Gold Standard analytical methods based on mass spectrometry (MS) and tandem mass spectrometry (MS/MS), i.e., GC-MS, GC-MS/MS, LC-MS and LC-MS/MS. Other chromatographic and non-chromatographic methods were also considered. The results for several different endogenous substances, including nitrate, anandamide, homoarginine, creatinine and malondialdehyde in human plasma, serum and urine are discussed. In addition to the Bland-Altman approach, linear regression analysis and the Oldham-Eksborg method-comparison approaches were used and compared. Special emphasis was given to the relation of difference and mean in the Bland-Altman approach. Currently available guidelines for method validation were also considered. Acceptance criteria for method agreement were proposed, including the slope and correlation coefficient in linear regression, and the coefficient of variation for the percentage difference in the Bland-Altman and Oldham-Eksborg approaches.
Keywords: Bland and Altman approach; Eksborg; Oldham; agreement; biomarkers; comparison; linear regression analysis; mass spectrometry; tandem mass spectrometry; validation.
Conflict of interest statement
The author declares no conflict of interest.
Figures












References
-
- Deming W.E. Statistical Adjustment of Data. John Wiley and Sons; New York, NY, USA: 1943. p. 184.
-
- Oldham P.D. Measurement in Medicine: The Interpretation of Numerical Data. English Universities Press; London, UK: 1968.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

