Phenylketonuria (PKU) Urinary Metabolomic Phenotype Is Defined by Genotype and Metabolite Imbalance: Results in 51 Early Treated Patients Using Ex Vivo 1H-NMR Analysis
- PMID: 37446577
- PMCID: PMC10343293
- DOI: 10.3390/molecules28134916
Phenylketonuria (PKU) Urinary Metabolomic Phenotype Is Defined by Genotype and Metabolite Imbalance: Results in 51 Early Treated Patients Using Ex Vivo 1H-NMR Analysis
Abstract
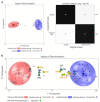
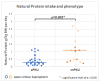
Phenylketonuria (PKU) is a rare metabolic disorder caused by mutations in the phenylalanine hydroxylase gene. Depending on the severity of the genetic mutation, medical treatment, and patient dietary management, elevated phenylalanine (Phe) may occur in blood and brain tissues. Research has recently shown that high Phe not only impacts the central nervous system, but also other organ systems (e.g., heart and microbiome). This study used ex vivo proton nuclear magnetic resonance (1H-NMR) analysis of urine samples from PKU patients (mean 14.9 ± 9.2 years, n = 51) to identify the impact of elevated blood Phe and PKU treatment on metabolic profiles. Our results found that 24 out of 98 urinary metabolites showed a significant difference (p < 0.05) for PKU patients compared to age-matched healthy controls (n = 51) based on an analysis of urinary metabolome. These altered urinary metabolites were related to Phe metabolism, dysbiosis, creatine synthesis or intake, the tricarboxylic acid (TCA) cycle, end products of nicotinamide-adenine dinucleotide degradation, and metabolites associated with a low Phe diet. There was an excellent correlation between the metabolome and genotype of PKU patients and healthy controls of 96.7% in a confusion matrix model. Metabolomic investigations may contribute to a better understanding of PKU pathophysiology.
Keywords: Ex Vivo 1H-NMR analysis spectroscopy; genotype; metabolomics; pathogenesis; phenylketonuria.
Conflict of interest statement
C.C., M.S. and M.G. are employees of Bruker Biospin, Ettlingen, Germany; otherwise, the authors declare no conflict of interest.
Figures









References
-
- Van Wegberg A.M.J., MacDonald A., Ahring K., Bélanger-Quintana A., Blau N., Bosch A.M., Burlina A., Campistol J., Feillet F., Giżewska M., et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017;12:162. doi: 10.1186/s13023-017-0685-2. - DOI - PMC - PubMed
-
- Pilotto A., Blau N., Leks E., Schulte C., Deuschl C., Zipser C., Piel D., Freisinger P., Gramer G., Kölker S., et al. Cerebrospinal fluid biogenic amines depletion and brain atrophy in adult patients with phenylketonuria. J. Inherit. Metab. Dis. 2019;42:398–406. doi: 10.1002/jimd.12049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

