Research on Electric Field-Induced Catalysis Using Single-Molecule Electrical Measurement
- PMID: 37446629
- PMCID: PMC10343440
- DOI: 10.3390/molecules28134968
Research on Electric Field-Induced Catalysis Using Single-Molecule Electrical Measurement
Abstract
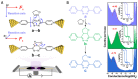
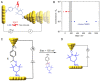
The role of catalysis in controlling chemical reactions is crucial. As an important external stimulus regulatory tool, electric field (EF) catalysis enables further possibilities for chemical reaction regulation. To date, the regulation mechanism of electric fields and electrons on chemical reactions has been modeled. The electric field at the single-molecule electronic scale provides a powerful theoretical weapon to explore the dynamics of individual chemical reactions. The combination of electric fields and single-molecule electronic techniques not only uncovers new principles but also results in the regulation of chemical reactions at the single-molecule scale. This perspective focuses on the recent electric field-catalyzed, single-molecule chemical reactions and assembly, and highlights promising outlooks for future work in single-molecule catalysis.
Keywords: chemical reactions; electric field catalysis; single-molecule electronic techniques.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Mehta P., Barboun P., Go D.B., Hicks J.C., Schneider W.F. Catalysis enabled by plasma activation of strong chemical bonds: A review. ACS Energy Lett. 2019;4:1115–1133. doi: 10.1021/acsenergylett.9b00263. - DOI
-
- Sun Z., Huang X.B., Zhang G. TiO2-based catalysts for photothermal catalysis: Mechanisms, materials and applications. J. Clean. Prod. 2022;381:135156–135174. doi: 10.1016/j.jclepro.2022.135156. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

