A Potential Anti-Glioblastoma Compound LH20 Induces Apoptosis and Arrest of Human Glioblastoma Cells via CDK4/6 Inhibition
- PMID: 37446710
- PMCID: PMC10343613
- DOI: 10.3390/molecules28135047
A Potential Anti-Glioblastoma Compound LH20 Induces Apoptosis and Arrest of Human Glioblastoma Cells via CDK4/6 Inhibition
Abstract
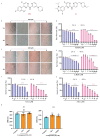
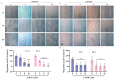
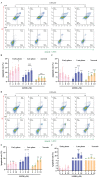
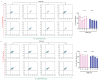
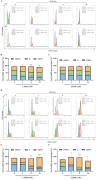
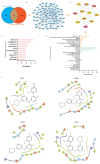
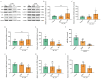
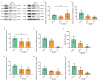
Glioblastoma (GBM) is a deadly brain tumor characterized by signaling dysregulation and aberrant cell cycle control. The CDK4/6-Rb axis is dysregulated in approximately 80% of all GBM cases. In this study, the anti-GBM effect of a novel pyrimidin-2-amine, LH20 was evaluated in vitro using the primary GBM cell lines U87MG and U251. GBM cells were administered LH20 at concentrations of 0.1, 1, 4, 8, 10, 20, 100, and 200 µM for 24 and 48 h, and the proliferation rate was evaluated using a CCK8 assay. Migration, apoptosis, and cell cycle were also assessed using a wound healing assay, Annexin V-FITC/PI apoptosis assay, and cell cycle staining, respectively. The targets of LH20 were predicted using SwissTargetPrediction and molecular docking. Western blotting analysis was performed to confirm the anti-GBM mechanism of LH20. We found that at concentrations of 4, 8, and 10 µM, LH20 significantly inhibited the proliferation and migration of U87MG and U251 cells, induced late phase apoptosis, promoted tumor cell necrosis, and arrested the G2/M phase of the cell cycle. LH20 also inhibited CDK4 and CDK6 activities by decreasing the phosphorylation of Rb. Our results suggest LH20 as a potential treatment strategy against GBM.
Keywords: CDKs; G2/M; LH20; apoptosis; glioblastoma; mitochondrial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

