Foegraecumoside O and P, a Pair of Triterpenoid Saponins with a 4/5/6 Fused Tricyclic Oleanane Carbon Skeleton from Lysimachia foenum-graecum Hance
- PMID: 37446727
- PMCID: PMC10343785
- DOI: 10.3390/molecules28135061
Foegraecumoside O and P, a Pair of Triterpenoid Saponins with a 4/5/6 Fused Tricyclic Oleanane Carbon Skeleton from Lysimachia foenum-graecum Hance
Abstract
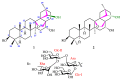
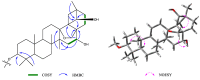
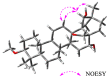
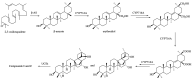
Lysimachia foenum-graecum Hance (Primulaceae) is a medicinal plant used for cold, pain, ascariasis, etc., in China. Triterpenoid saponins have been found to be the main components of this genus. In this work, a pair of oleanane-type triterpenoid saponins with an unprecedented 4/5/6 fused tricyclic skeleton, foegraecumoside O (1) and foegraecumoside P (2) were isolated from the butanol fraction of the aerial parts of L. foenum-graecum. Their structures were determined using chemical methods and extensive spectroscopic analyses, along with quantum chemical calculations. Compound 2 displayed moderate cytotoxicity against HepG2, MGC-803, T24, NCI-H460, A549, and A549/CDDP (drug-resistant lung-cancer cell line) with IC50 at 12.4-19.2 μM in an MTT assay, comparing with the positive control doxorubicin, which had IC50 at 0.53-4.92 μM, but was inactive for A549/CDDP. Furthermore, a possible biosynthetic pathway for forming compounds 1 and 2 was proposed.
Keywords: Lysimachia; Lysimachia foenum-graecum; Primulaceae; cytotoxicity; triterpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cytotoxic triterpenoid saponins from Lysimachia foenum-graecum.Phytochemistry. 2017 Apr;136:165-174. doi: 10.1016/j.phytochem.2017.01.021. Epub 2017 Feb 5. Phytochemistry. 2017. PMID: 28173950
-
Two new triterpenes from Lysimachia foenum-graecum.J Asian Nat Prod Res. 2009 Jun;11(6):529-33. doi: 10.1080/10286020902930884. J Asian Nat Prod Res. 2009. PMID: 20183286
-
Two triterpenes from Lysimachia foenum-graecum.J Asian Nat Prod Res. 2009;11(2):128-31. doi: 10.1080/10286020802573859. J Asian Nat Prod Res. 2009. PMID: 19219724
-
Two new triterpenes from Lysimachia foenum-graecum.J Asian Nat Prod Res. 2010 Mar;12(3):204-8. doi: 10.1080/10286020903523258. J Asian Nat Prod Res. 2010. PMID: 20390766
-
Oleanane-type triterpenoid saponins from Lysimachia fortunei Maxim.Phytochemistry. 2018 Mar;147:140-146. doi: 10.1016/j.phytochem.2017.12.022. Epub 2018 Jan 8. Phytochemistry. 2018. PMID: 29324278
Cited by
-
Clerodane Furanoditerpenoids from Tinospora bakis (A.Rich.) Miers (Menispermaceae).Molecules. 2023 Dec 26;29(1):154. doi: 10.3390/molecules29010154. Molecules. 2023. PMID: 38202737 Free PMC article.
References
-
- Hu C., Kelso S. Primulaceae. Flora China. 1996;5:39–189.
-
- Committee of Chinese Materia Medica. National Administration of Traditional Chinese Medicine . Chinese Materia Medica. Shanghai Science and Technology Press; Shanghai, China: 1999. Lysimachia foenum-graecum Hance; pp. 5364–5366.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

