L-Rhamnose and Phenolic Esters-Based Monocatenar and Bolaform Amphiphiles: Eco-Compatible Synthesis and Determination of Their Antioxidant, Eliciting and Cytotoxic Properties
- PMID: 37446816
- PMCID: PMC10343848
- DOI: 10.3390/molecules28135154
L-Rhamnose and Phenolic Esters-Based Monocatenar and Bolaform Amphiphiles: Eco-Compatible Synthesis and Determination of Their Antioxidant, Eliciting and Cytotoxic Properties
Abstract
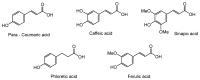
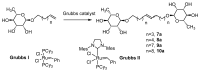
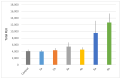
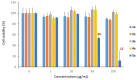
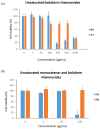
Symmetrical and dissymmetrical bolaforms were prepared with good to high yields from unsaturated L-rhamnosides and phenolic esters (ferulic, phloretic, coumaric, sinapic and caffeic) using two eco-compatible synthetic strategies involving glycosylation, enzymatic synthesis and cross-metathesis under microwave activation. The plant-eliciting activity of these new compounds was investigated in Arabidopsis model plants. We found that the monocatenar rhamnosides and bolaforms activate the plant immune system with a response depending on the carbon chain length and the nature of the hydrophilic heads. Their respective antioxidant activities were also evaluated, as well as their cytotoxic properties on dermal cells for cosmetic uses. We showed that phenolic ester-based compounds present good antioxidant activities and that their cytotoxicity is low. These properties are also dependent on the carbon chains used.
Keywords: amphiphilic; antioxidant; bolaform; dermal cytotoxicity; microwaves; monocatenar; phenolic acids; plant immunity; rhamnosides.
Conflict of interest statement
The author declare no conflict of interest.
Figures
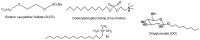
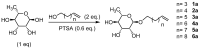





References
-
- Ash I., Ash M. Encyclopedia of Surfactants. Chemical Pub. Co.; New York, NY, USA: 1983.
-
- Gonçalves R.A., Holmberg K., Lindman B. Cationic surfactants: A review. J. Mol. Liq. 2023;375:121335–121346. doi: 10.1016/j.molliq.2023.121335. - DOI
-
- Sarkar R., Pal A., Rakshit A., Saha B. Properties and applications of amphoteric surfactant: A concise review. J. Surfactants Deterg. 2021;24:709–730. doi: 10.1002/jsde.12542. - DOI
-
- Singh N., Sharma L. Synthesis of carbohydrate derived non-ionic gemini surfactants and study of their micellar and reverse micellar behavior—A review. Lett. Org. Chem. 2019;16:607–614. doi: 10.2174/1570178616666190123124727. - DOI
-
- Zhou M., Li S., Zhang Z., Wang C., Luo G., Zhao J. Progress in the Synthesis of Zwitterionic Gemini Surfactants. J. Surfactants Deterg. 2017;20:1243–12541. doi: 10.1007/s11743-017-2014-0. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

