Uniformly Dispersed Sb-Nanodot Constructed by In Situ Confined Polymerization of Ionic Liquids for High-Performance Potassium-Ion Batteries
- PMID: 37446874
- PMCID: PMC10343779
- DOI: 10.3390/molecules28135212
Uniformly Dispersed Sb-Nanodot Constructed by In Situ Confined Polymerization of Ionic Liquids for High-Performance Potassium-Ion Batteries
Abstract
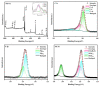
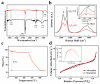
Antimony (Sb) is a potential candidate anode for potassium-ion batteries (PIBs) owing to its high theoretical capacity. However; in the process of potassium alloying reaction; the huge volume expansion (about 407%) leads to pulverization of active substance as well as loss of electrical contact resulting in rapidly declining capacity. Herein; uniformly dispersed Sb-Nanodot in carbon frameworks (Sb-ND@C) were constructed by in situ confined polymerization of ionic liquids. Attributed to the uniformly dispersed Sb-ND and confinement effect of carbon frameworks; as anode for PIBs; Sb-ND@C delivered a superior rate capability (320.1 mA h g-1 at 5 A g-1) and an outstanding cycling stability (486 mA h g-1 after 1000 cycles; achieving 89.8% capacity retention). This work offers a facile route to prepare highly dispersed metal-Nanodot via the in situ polymerization of ionic liquid for high-performance metal-ion batteries.
Keywords: Sb; anode; ionic liquids; nanodot; potassium-ion batteries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Li D., Zhu M., Chen L., Chen L., Zhai W., Ai Q., Hou G., Sun Q., Liu Y., Liang Z., et al. Sandwich-Like FeCl3@C as High-Performance Anode Materials for Potassium-Ion Batteries. Adv. Mater. Interfaces. 2018;5:1800606. doi: 10.1002/admi.201800606. - DOI
-
- Pramudita J.C., Sehrawat D., Goonetilleke D., Sharma N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017;7:1602911. doi: 10.1002/aenm.201602911. - DOI
-
- Tang M., Wu Y., Chen Y., Jiang C., Zhu S., Zhuo S., Wang C. An Organic Cathode with High Capacities for Fast-Charge Potassium-Ion Batteries. J. Mater. Chem. A. 2019;7:486–492. doi: 10.1039/C8TA09960J. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

