Iodine(V)-Based Oxidants in Oxidation Reactions
- PMID: 37446912
- PMCID: PMC10343256
- DOI: 10.3390/molecules28135250
Iodine(V)-Based Oxidants in Oxidation Reactions
Abstract
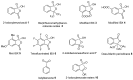
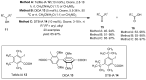
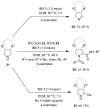
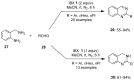
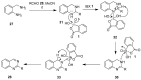
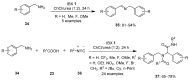
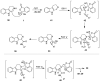
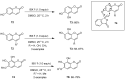
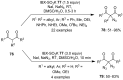
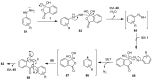
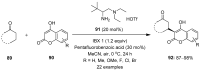
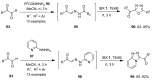
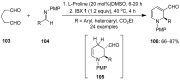
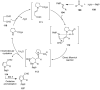
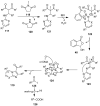
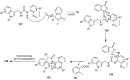
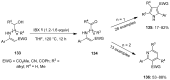
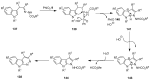
The chemistry of hypervalent iodine reagents has now become quite valuable due to the reactivity of these compounds under mild reaction conditions and their resemblance in chemical properties to transition metals. The environmentally friendly nature of these reagents makes them suitable for Green Chemistry. Reagents with a dual nature, such as iodine(III) reagents, are capable electrophiles, while iodine(V) reagents are known for their strong oxidant behavior. Various iodine(V) reagents including IBX and DMP have been used as oxidants in organic synthesis either in stoichiometric or in catalytic amounts. In this review article, we describe various oxidation reactions induced by iodine(V) reagents reported in the past decade.
Keywords: catalyst; hypervalent iodine(V) reagents; oxidant; oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



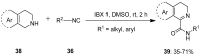






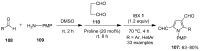
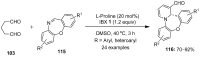

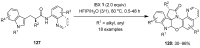

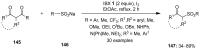
Similar articles
-
Palladium-Catalyzed Organic Reactions Involving Hypervalent Iodine Reagents.Molecules. 2022 Jun 17;27(12):3900. doi: 10.3390/molecules27123900. Molecules. 2022. PMID: 35745020 Free PMC article. Review.
-
Hypervalent iodine reagents as a new entrance to organocatalysts.Chem Commun (Camb). 2009 Apr 28;(16):2073-85. doi: 10.1039/b821747e. Epub 2009 Mar 11. Chem Commun (Camb). 2009. PMID: 19360157
-
Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry.Molecules. 2017 May 12;22(5):780. doi: 10.3390/molecules22050780. Molecules. 2017. PMID: 28498333 Free PMC article. Review.
-
Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions.Chem Asian J. 2014 Apr;9(4):950-71. doi: 10.1002/asia.201301582. Epub 2014 Feb 12. Chem Asian J. 2014. PMID: 24523252
-
Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions.Front Chem. 2020 Sep 29;8:705. doi: 10.3389/fchem.2020.00705. eCollection 2020. Front Chem. 2020. PMID: 33134246 Free PMC article. Review.
Cited by
-
Recent advances in transition-metal-free arylation reactions involving hypervalent iodine salts.Beilstein J Org Chem. 2024 Nov 13;20:2891-2920. doi: 10.3762/bjoc.20.243. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39559439 Free PMC article. Review.
-
Catalyst-Free Trans-Selective Oxyiodination and Oxychlorination of Alkynes Employing N-X (Halogen) Reagents.Molecules. 2023 Nov 3;28(21):7420. doi: 10.3390/molecules28217420. Molecules. 2023. PMID: 37959838 Free PMC article.
-
meso-Tetrahexyl-7,8-dihydroxychlorin and Its Conversion to ß-Modified Derivatives.Molecules. 2024 May 5;29(9):2144. doi: 10.3390/molecules29092144. Molecules. 2024. PMID: 38731635 Free PMC article.
References
-
- Wirth T., editor. Hypervalent Iodine Chemistry Modern Developments in Organic Synthesis. Springer; Berlin/Heidelberg, Germany: 2003.
-
- Zhdankin V.V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds. Volume I Wiley; Chichester, UK: 2013.
-
- Singh F.V. In: Thomas Wirth, in Comprehensive Organic Synthesis II. Knochel P., Molander G., editors. Elsevier; Amsterdam, The Netherlands: 2014. p. 880.
-
- Singh F.V., Wirth T. In: Science of Synthesis: Catalytic Oxidation in Organic Synthesis. Muñiz K., editor. Thieme; Stuttgart, Germany: 2017. p. 29.
-
- Singh F.V., Wirth T. In: The Chemistry of Hypervalent Halogen Compounds. Olofsson B., Ilan M., Zvi R., editors. John Wiley & Sons, Ltd.; Chichester, UK: 2018. p. 809.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

