(1-(4-(5-Phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1 H-1,2,3-triazol-4-yl)-methylenyls α,ω-Bisfunctionalized 3- and 4-PEG: Synthesis and Photophysical Studies
- PMID: 37446917
- PMCID: PMC10343605
- DOI: 10.3390/molecules28135256
(1-(4-(5-Phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1 H-1,2,3-triazol-4-yl)-methylenyls α,ω-Bisfunctionalized 3- and 4-PEG: Synthesis and Photophysical Studies
Abstract
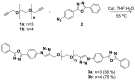
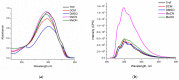
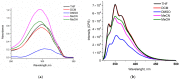
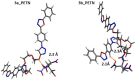
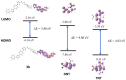
Two new azaheterocycle-based bolas, such as (1-(4-(5-phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1H-1,2,3-triazol-4-yl)-methylenyls α,ω-bisfunctionalized PEGs, were prepared via Cu-catalyzed click reaction between 2-(4-azidophenyl)-5-(aryl)-oxadiazole-1,3,4 and terminal ethynyls derived from PEG-3 and PEG-4. Due to the presence of two heteroaromatic cores and a PEG linker, these bola molecules are considered as promising fluorescent chemosensors for electron-deficient species. As a result of a well-pronounced "turn-off" fluorescence response towards common nitro-explosive components, such as 2,4-dinitrotoluene (DNT) and 2,4,6-trinitrotoluene (TNT), hard-to-detect pentaerythritol tetranitrate (PETN), as well as Hg2+ cation was observed.
Keywords: Hg2+; PEGs; bola molecules; fluorescence; fluorescence quenching; nitro-explosive components; oxadiazole; pentaerythritol tetranitrate; sensor; “click” reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities.Int J Mol Sci. 2023 Jun 13;24(12):10084. doi: 10.3390/ijms241210084. Int J Mol Sci. 2023. PMID: 37373234 Free PMC article.
-
Transformation of TNT, 2,4-DNT, and PETN by Raoultella planticola M30b and Rhizobium radiobacter M109 and exploration of the associated enzymes.World J Microbiol Biotechnol. 2020 Nov 28;36(12):190. doi: 10.1007/s11274-020-02962-8. World J Microbiol Biotechnol. 2020. PMID: 33247357
-
Detection of explosives by positive corona discharge ion mobility spectrometry.J Hazard Mater. 2010 Apr 15;176(1-3):692-6. doi: 10.1016/j.jhazmat.2009.11.087. Epub 2009 Dec 8. J Hazard Mater. 2010. PMID: 20004055
-
UV-FIA: UV-induced fluoro-immunochemical assay for ultra-trace detection of PETN, RDX, and TNT.Anal Chim Acta. 2019 Oct 24;1077:266-272. doi: 10.1016/j.aca.2019.05.048. Epub 2019 May 23. Anal Chim Acta. 2019. PMID: 31307718
-
Computer vision vs. spectrofluorometer-assisted detection of common nitro-explosive components with bola-type PAH-based chemosensors.RSC Adv. 2021 Jul 27;11(42):25850-25857. doi: 10.1039/d1ra03108b. eCollection 2021 Jul 27. RSC Adv. 2021. PMID: 35479431 Free PMC article.
References
-
- Escamilla G.H., Newkome G.R. Organic Synthesis Highlights III. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2008. Bolaamphiphiles: Golf balls to fibers; pp. 382–390. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

