Experimental and Theoretical Estimations of Atrazine's Adsorption in Mangosteen-Peel-Derived Nanoporous Carbons
- PMID: 37446931
- PMCID: PMC10343179
- DOI: 10.3390/molecules28135268
Experimental and Theoretical Estimations of Atrazine's Adsorption in Mangosteen-Peel-Derived Nanoporous Carbons
Abstract
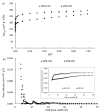
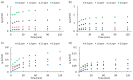
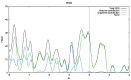
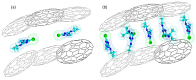
Nanoporous carbons were prepared via chemical and physical activation from mangosteen-peel-derived chars. The removal of atrazine was studied due to the bifunctionality of the N groups. Pseudo-first-order, pseudo-second-order, and intraparticle pore diffusion kinetic models were analyzed. Adsorption isotherms were also analyzed according to the Langmuir and Freundlich models. The obtained results were compared against two commercially activated carbons with comparable surface chemistry and porosimetry. The highest uptake was found for carbons with higher content of basic surface groups. The role of the oxygen-containing groups in the removal of atrazine was estimated experimentally using the surface density. The results were compared with the adsorption energy of atrazine theoretically estimated on pristine and functionalized graphene with different oxygen groups using periodic DFT methods. The energy of adsorption followed the same trend observed experimentally, namely the more basic the pH, the more favored the adsorption of atrazine. Micropores played an important role in the uptake of atrazine at low concentrations, but the presence of mesoporous was also required to inhibit the pore mass diffusion limitations. The present work contributes to the understanding of the interactions between triazine-based pollutants and the surface functional groups on nanoporous carbons in the liquid-solid interface.
Keywords: DFT estimations; atrazine removal; isotherms; kinetics; nanoporous carbons.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Sustainable production of nanoporous carbons: Kinetics and equilibrium studies in the removal of atrazine.J Colloid Interface Sci. 2020 Mar 7;562:252-267. doi: 10.1016/j.jcis.2019.12.026. Epub 2019 Dec 9. J Colloid Interface Sci. 2020. PMID: 31838361
-
The Use of High Surface Area Mesoporous-Activated Carbon from Longan Seed Biomass for Increasing Capacity and Kinetics of Methylene Blue Adsorption from Aqueous Solution.Molecules. 2021 Oct 28;26(21):6521. doi: 10.3390/molecules26216521. Molecules. 2021. PMID: 34770928 Free PMC article.
-
Sorption of atrazine on conventional and surface modified activated carbons.J Colloid Interface Sci. 2006 Oct 15;302(2):408-16. doi: 10.1016/j.jcis.2006.06.065. Epub 2006 Jul 8. J Colloid Interface Sci. 2006. PMID: 16870200
-
Removal Effect of Atrazine in Co-Solution with Bisphenol A or Humic Acid by Different Activated Carbons.Materials (Basel). 2018 Dec 16;11(12):2558. doi: 10.3390/ma11122558. Materials (Basel). 2018. PMID: 30558368 Free PMC article.
-
From slit pores to 3D frameworks: Advances in molecular modeling of adsorption in nanoporous carbons.Adv Colloid Interface Sci. 2025 Aug;342:103502. doi: 10.1016/j.cis.2025.103502. Epub 2025 Apr 4. Adv Colloid Interface Sci. 2025. PMID: 40239421 Review.
References
-
- Ratnayaka D.D., Brandt M.J., Johnson K.M. Specialized and advanced water treatment processes. In: Ratnayaka D., Brandt M.J., Johnson K.M., editors. Water Supply. 6th ed. Elsevier; Amsterdam, The Netherlands: 2009. pp. 365–423.
-
- Crittenden J.C., Trussell R.R., Hand D.W., Howe K.J., Tchobanoglous G. MWH’s Water Treatment: Principles and Design. John and Wiley and Sons; Hoboken, NJ, USA: 2012. Granular filtration; pp. 727–818.
Grants and funding
LinkOut - more resources
Full Text Sources

