The Sweet Potato K+ Transporter IbHAK11 Regulates K+ Deficiency and High Salinity Stress Tolerance by Maintaining Positive Ion Homeostasis
- PMID: 37446983
- PMCID: PMC10346689
- DOI: 10.3390/plants12132422
The Sweet Potato K+ Transporter IbHAK11 Regulates K+ Deficiency and High Salinity Stress Tolerance by Maintaining Positive Ion Homeostasis
Abstract
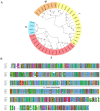
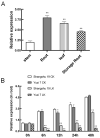
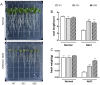
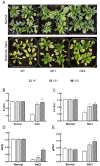
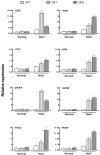
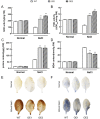
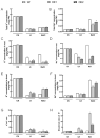
The K+ transporter KT/HAK/KUP (K+ transporter/high-affinity K+/K+ uptake) family has a critical effect on K+ uptake and translocation in plants under different environmental conditions. However, the functional analysis of KT/HAK/KUP members in sweet potatoes is still limited. The present work reported the physiological activity of a new gene, IbHAK11, in the KT/HAK/KUP family in sweet potatoes. IbHAK11 expression increased significantly in the low K+-tolerant line compared with the low K+-sensitive line following treatment with low K+ concentrations. IbHAK11 upregulation promoted root growth in Arabidopsis under low K+ conditions. Under high saline stress, transgenic lines had superior growth and photosynthetic characteristics compared with the wild-type (WT). As for IbHAK11-overexpressing plants, activation of both the non-enzymatic and enzymatic reactive oxygen species (ROS) scavenging systems was observed. Therefore, IbHAK11-overexpressing plants had lower malondialdehyde (MDA) and ROS levels (including H2O2 and O2-) compared with WT under salt-induced stress. We also found that under both low K+ and high salinity conditions, overexpression of IbHAK11 enhanced K+ translocation from the root to the shoot and decreased Na+ absorption in Arabidopsis. Consequently, IbHAK11 positively regulated K+ deficiency and high salinity stresses by regulating K+ translocation and Na+ uptake, thus maintaining K+/Na+ homeostasis in plants.
Keywords: IbHAK11; K+ deficiency tolerance; K+ transporter; K+/Na+ homeostasis; high salinity tolerance; sweet potato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kul R. Integrated application of plant growth promoting rhizobacteria and biochar improves salt tolerance in eggplant seedlings. Turk. J. Agric. For. 2022;46:677–702. doi: 10.55730/1300-011X.3035. - DOI
-
- Addique M., Kausar A., Iqra I., Akhter N., Mujahid N., Parveen A., Zaman Q., Hussain S. Amino acids application alleviated salinity stress in spinach (Spinacia oleracea L.) by improving oxidative defense, osmolyte accumulation, and nutrient balance. Turk. J. Agric. For. 2022;46:875–887. doi: 10.55730/1300-011X.3049. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

