Photosynthesis in the Biomass Model Species Lemna minor Displays Plant-Conserved and Species-Specific Features
- PMID: 37447003
- PMCID: PMC10361204
- DOI: 10.3390/plants12132442
Photosynthesis in the Biomass Model Species Lemna minor Displays Plant-Conserved and Species-Specific Features
Abstract
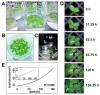
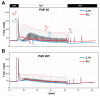
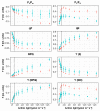
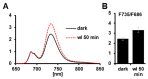
Lemnaceae are small freshwater plants with extraordinary high growth rates. We aimed to test whether this correlates with a more efficient photosynthesis, the primary energy source for growth. To this end, we compared photosynthesis properties of the duckweed Lemna minor and the terrestrial model plant Arabidopsis thaliana. Chlorophyll fluorescence analyses revealed high similarity in principle photosynthesis characteristics; however, Lemna exhibited a more effective light energy transfer into photochemistry and more stable photosynthesis parameters especially under high light intensities. Western immunoblot analyses of representative photosynthesis proteins suggested potential post-translational modifications in Lemna proteins that are possibly connected to this. Phospho-threonine phosphorylation patterns of thylakoid membrane proteins displayed a few differences between the two species. However, phosphorylation-dependent processes in Lemna such as photosystem II antenna association and the recovery from high-light-induced photoinhibition were not different from responses known from terrestrial plants. We thus hypothesize that molecular differences in Lemna photosynthesis proteins are associated with yet unidentified mechanisms that improve photosynthesis and growth efficiencies. We also developed a high-magnification video imaging approach for Lemna multiplication which is useful to assess the impact of external factors on Lemna photosynthesis and growth.
Keywords: Arabidopsis thaliana; Lemna minor; photoinhibition; photosynthesis; photosystem antenna; post-translational modifications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bog M., Appenroth K.-J., Sree K.S. Duckweed (Lemnaceae): Its Molecular Taxonomy. Front. Sustain. Food Syst. 2019;3:117. doi: 10.3389/fsufs.2019.00117. - DOI
-
- Acosta K., Appenroth K.J., Borisjuk L., Edelman M., Heinig U., Jansen M.A.K., Oyama T., Pasaribu B., Schubert I., Sorrels S., et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell. 2021;33:3207–3234. doi: 10.1093/plcell/koab189. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

