Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato
- PMID: 37447072
- PMCID: PMC10347230
- DOI: 10.3390/plants12132512
Identification and Expression of the CorA/MRS2/ALR Type Magnesium Transporters in Tomato
Abstract
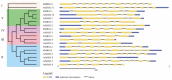
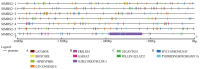
Magnesium (Mg2+) is the most abundant divalent ion in plants, participating in numerous metabolic processes in growth and development. CorA/MRS2/ALR type Mg2+ transporters are essential for maintaining Mg2+ homeostasis in plants. However, the candidate protein and its potential functions in the tomato plant have not been fully understood. In this study, we identified seven MGT genes (SlMRS2) in tomato based on sequence similarity, domain analysis, conserved motif identification, and structure prediction. Two SlMRS2 genes were analyzed in the bacterial strain MM281, and a functional complementary assay demonstrated their high-affinity transport of Mg2+. Quantitative real-time PCR analysis revealed that the expressions of these Mg2+ transporters were down-regulated in leaves under Mg2+ limitation, with a greater impact on lower and middle leaves compared to young leaves. Conversely, under Mg2+ toxicity, several genes were up-regulated in leaves with a circadian rhythm. Our findings indicate that members of the SlMRS2 family function as Mg2+ transporters and lay the groundwork for further analysis of their distinct functions in tomato.
Keywords: RT-PCR; genomic analysis; magnesium transporter; qRT-PCR; tomato (Solanum lycopersicum L.).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



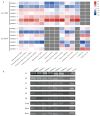

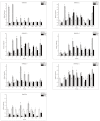

References
-
- Marschner H. Mineral Nutrition of Higher Plants. 3rd ed. Academic Press; San Diego, CA, USA: 2012. p. 672. - DOI
-
- Tunc-Ozdemir M., Tang C., Ishka M.R., Brown E., Groves N.R., Myers C.T., Rato C., Poulsen L.R., McDowell S., Miller G., et al. A Cyclic Nucleotide-Gated Channel (CNGC16) in Pollen Is Critical for Stress Tolerance in Pollen Reproductive Development. Plant Physiol. 2013;161:1010–1020. doi: 10.1104/pp.112.206888. - DOI - PMC - PubMed
-
- Guo W.L., Nazim H., Liang Z.S., Yang D.F. Magnesium deficiency in plants: An urgent problem. Crop J. 2016;4:83–91. doi: 10.1016/j.cj.2015.11.003. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

