Pharmacokinetic Analyses of Liposomal and Non-Liposomal Multivitamin/Mineral Formulations
- PMID: 37447400
- PMCID: PMC10347199
- DOI: 10.3390/nu15133073
Pharmacokinetic Analyses of Liposomal and Non-Liposomal Multivitamin/Mineral Formulations
Abstract
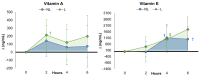
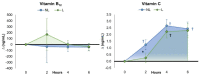
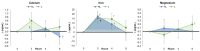
Recent research supports previous contentions that encapsulating vitamins and minerals with liposomes help improve overall bioavailability. This study examined whether ingesting a liposomal multivitamin and mineral supplement (MVM) differentially affects the appearance and/or clearance of vitamins and minerals in the blood compared to a non-liposomal MVM supplement. In a double-blind, randomized, and counterbalanced manner, 34 healthy men and women fasted for 12 h. Then, they ingested a non-liposomal (NL) or liposomal (L) MVM supplement and a standardized snack. Venous blood samples were obtained at 0, 2, 4, and 6 h after MVM ingestion and analyzed for a panel of vitamins and minerals. Plasma levels of vitamins and minerals and mean changes from baseline with 95% confidence intervals (CIs) were analyzed using general linear model statistics with repeated measures. The observed values were also entered into pharmacokinetic analysis software and analyzed through univariate analysis of variance with repeated measure contrasts. The results revealed an overall treatment x time interaction effect among the vitamins and minerals evaluated (p = 0.051, ηp2 = 0.054, moderate effect). Differences between treatments were also observed in volume distribution area (vitamin E, iron), median residence time (vitamin E, iron), volume distribution area (iron), volume of distribution steady state (vitamin A, E, iron), clearance rates (vitamin A, E), elimination phase half-life (vitamin E, iron), distribution/absorption phase intercept (vitamin A), and distribution/absorption phase slope and rate (vitamin C, calcium). Vitamin volume distribution was lower with liposomal MVM ingestion than non-liposomal MVM sources, suggesting greater clearance and absorption since similar amounts of vitamins and minerals were ingested. These findings indicate that coating a MVM with liposomes affects individual nutrient pharmacokinetic profiles. Additional research should evaluate how long-term supplementation of liposomal MVM supplements may affect vitamin and mineral status, nutrient function, and/or health outcomes.
Keywords: bioavailability; calcium; iron; magnesium; nutrient absorption; vitamin A; vitamin B12; vitamin C; vitamin E.
Conflict of interest statement
R.B.K. has conducted sponsored research on nutritional supplements through grants and contracts awarded to the universities he has been affiliated with, received honorarium for presenting research related to dietary supplements, served as an expert on cases related to dietary supplements, and consulted with industry on product development unrelated to the nutrient studied in the present investigation. Other authors report no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

