Chitosan-Collagen Electrospun Nanofibers Loaded with Curcumin as Wound-Healing Patches
- PMID: 37447576
- PMCID: PMC10347256
- DOI: 10.3390/polym15132931
Chitosan-Collagen Electrospun Nanofibers Loaded with Curcumin as Wound-Healing Patches
Abstract
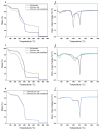
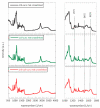
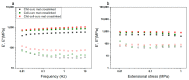
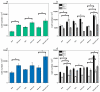
Composite chitosan-collagen nanofibrous mats embedded with curcumin were prepared via a single-step electrospinning procedure and explored as wound-healing patches with superior biological activity. A mild crosslinking protocol consisting of a short exposure to ammonia vapor and UV radiation was developed to ensure proper stability in physiological-like conditions without affecting the intrinsic biocompatibility of chitosan and collagen. The fabricated composite patches displayed a highly porous, homogeneous nanostructure consisting of fibers with an average diameter of 200 nm, thermal stability up to 200 °C, mechanical features able to ensure protection and support to the new tissues, and water-related properties in the ideal range to allow exudate removal and gas exchange. The release kinetic studies carried out in a simulated physiological environment demonstrated that curcumin release was sustained for 72 h when the mats are crosslinked hence providing prolonged bioactivity reflected by the displayed antioxidant properties. Remarkably, combining chitosan and collagen not only ensures prolonged stability and optimal physical-chemical properties but also allows for better-promoting cell adhesion and proliferation and enhanced anti-bacteriostatic capabilities with the addition of curcumin, owing to its beneficial anti-inflammatory effect, ameliorating the attachment and survival/proliferation rates of keratinocytes and fibroblasts to the fabricated patches.
Keywords: chitosan; collagen; curcumin; electrospun nanofibers; wound-healing patches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mastroianni M., Ng Z.Y., Goyal R., Mallard C., Farkash E.A., Leonard D.A., Albritton A., Shanmugarajah K., Kurtz J.M., Sachs D.H., et al. Topical Delivery of Immunosuppression to Prolong Xenogeneic and Allogeneic Split-Thickness Skin Graft Survival. J. Burn Care Res. 2018;39:363–373. doi: 10.1097/BCR.0000000000000597. - DOI - PMC - PubMed
-
- Dong R., Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290. doi: 10.1016/j.nantod.2021.101290. - DOI
-
- Rezvani Ghomi E., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., Ramakrishna S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019;136:47738. doi: 10.1002/app.47738. - DOI
LinkOut - more resources
Full Text Sources

