Imlifidase for Kidney Transplantation of Highly Sensitized Patients With a Positive Crossmatch: The French Consensus Guidelines
- PMID: 37448448
- PMCID: PMC10336835
- DOI: 10.3389/ti.2023.11244
Imlifidase for Kidney Transplantation of Highly Sensitized Patients With a Positive Crossmatch: The French Consensus Guidelines
Abstract
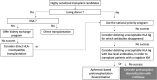
Imlifidase recently received early access authorization for highly sensitized adult kidney transplant candidates with a positive crossmatch against an ABO-compatible deceased donor. These French consensus guidelines have been generated by an expert working group, in order to homogenize patient selection, associated treatments and follow-up. This initiative is part of an international effort to analyze properly the benefits and tolerance of this new costly treatment in real-life. Eligible patients must meet the following screening criteria: cPRA ≥ 98%, ≤ 65-year of age, ≥ 3 years on the waiting list, and a low risk of biopsy-related complications. The final decision to use Imlifidase will be based on the two following criteria. First, the results of a virtual crossmatch on recent serum, which shall show a MFI for the immunodominant donor-specific antibodies (DSA) > 6,000 but the value of which does not exceed 5,000 after 1:10 dilution. Second, the post-Imlifidase complement-dependent cytotoxicity crossmatch must be negative. Patients treated with Imlifidase will receive an immunosuppressive regimen based on steroids, rATG, high dose IVIg, rituximab, tacrolimus and mycophenolic acid. Frequent post-transplant testing for DSA and systematic surveillance kidney biopsies are highly recommended to monitor post-transplant DSA rebound and subclinical rejection.
Keywords: desensitization; highly sensitized patients; imlifidase; kidney transplantation; positive crossmatch.
Copyright © 2023 Couzi, Malvezzi, Amrouche, Anglicheau, Blancho, Caillard, Freist, Guidicelli, Kamar, Lefaucheur, Mariat, Koenig, Noble, Thaunat, Thierry, Taupin and Bertrand.
Conflict of interest statement
LC has received lecture fees from Astellas, Chiesi, Novartis, Sandoz, Ostuka, GSK, Biotest, and participated on advisory boards for Biotest, Hansa, and Novartis. LA, DA, GG, CL, AK, J-LT, JN, and DB participated on advisory boards for Hansa. NK has received speakers fees from, and participated on advisory boards for Astellas, AstraZeneca, Biotest, CSL Behring, Chiesi, ExeViR, Hansa, Merck Sharp and Dohme, Glasgow Smith Kline, Novartis Pharma, Sanofi, Sandoz, and Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Comment in
-
Prime Time for HLA Desensitization: Imlifidase in the Spotlight.Transpl Int. 2023 Jun 28;36:11616. doi: 10.3389/ti.2023.11616. eCollection 2023. Transpl Int. 2023. PMID: 37456683 Free PMC article. No abstract available.
References
-
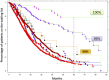
- Jordan SC, Legendre C, Desai NM, Lorant T, Bengtsson M, Lonze BE, et al. Imlifidase Desensitization in Crossmatch-Positive, Highly Sensitized Kidney Transplant Recipients: Results of an International Phase 2 Trial (Highdes). Transplantation (2021) 105(8):1808–17. 10.1097/tp.0000000000003496 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

