Contemporary Monoclonal Antibody Utilization in Glomerular Diseases
- PMID: 37448529
- PMCID: PMC10338194
- DOI: 10.1016/j.mayocpiqo.2023.04.009
Contemporary Monoclonal Antibody Utilization in Glomerular Diseases
Abstract
Therapeutic monoclonal antibodies (MAbs) have been one of the fastest growing drug classes in the past 2 decades and are indicated in the treatment of cancer, autoimmune disorders, solid organ transplantation, and glomerular diseases. The Food and Drug Administration has approved 100 MAbs between 1986 and 2021, and MAbs account for 20% of Food and Drug Administration's new drug approval every year. MAbs are preferred over traditional immunosuppressive agents because of their high specificity, reduced number of drug-drug interactions, and low toxicity, which make them a prime example of personalized medicine. In this review article, we provide an overview of the taxonomy, pharmacology, and therapeutic applications of MAbs in glomerular diseases. We searched the literature through PubMed using the following search terms: monoclonal antibodies, glomerular diseases, pharmacokinetics, pharmacodynamics, immunoglobulin, murine, chimeric,humanized, and fully human, and limited our search to years 2018-2023. We selected peer-reviewed journal articles with an evidence-based approach, prioritizing randomized control trials in specific glomerular diseases, if available. Advances in the MAb field have resulted in a significant paradigm shift in targeted treatment of immune-mediated glomerular diseases, and multiple randomized control trials are currently being conducted. Increased recognition is critical to expand their use in experimental research and personalized medicine.
© 2023 The Authors.
Conflict of interest statement
The authors report no competing interests.
Figures
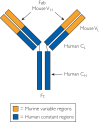
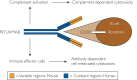
References
-
- Cosimi A.B. Clinical development of Orthoclone OKT3. Transplant Proc. 1987;19(2 Suppl 1):7–16. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

