Co-occurrence of marine and freshwater phycotoxins in oysters, and analysis of possible predictors for management
- PMID: 37448555
- PMCID: PMC10336265
- DOI: 10.1016/j.toxcx.2023.100166
Co-occurrence of marine and freshwater phycotoxins in oysters, and analysis of possible predictors for management
Abstract
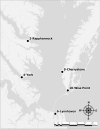
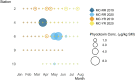
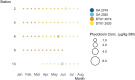
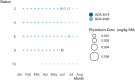
Oysters (Crassostrea virginica) were screened for 12 phycotoxins over two years in nearshore waters to collect baseline phycotoxin data and to determine prevalence of phycotoxin co-occurrence in the commercially and ecologically-relevant species. Trace to low concentrations of azaspiracid-1 and -2 (AZA1, AZA2), domoic acid (DA), okadaic acid (OA), and dinophysistoxin-1 (DTX1) were detected, orders of magnitude below seafood safety action levels. Microcystins (MCs), MC-RR and MC-YR, were also found in oysters (maximum: 7.12 μg MC-RR/kg shellfish meat wet weight), warranting consideration of developing action levels for freshwater phycotoxins in marine shellfish. Oysters contained phycotoxins that impair shellfish health: karlotoxin1-1 and 1-3 (KmTx1-1, KmTx1-3), goniodomin A (GDA), and pectenotoxin-2 (PTX2). Co-occurrence of phycotoxins in oysters was common (54%, n = 81). AZAs and DA co-occurred most frequently of the phycotoxins investigated that are a concern for human health (n = 13) and PTX2 and KmTxs co-occurred most frequently amongst the phycotoxins of concern for shellfish health (n = 9). Various harmful algal bloom (HAB) monitoring methods and tools were assessed for their effectiveness at indicating levels of phycotoxins in oysters. These included co-deployed solid phase adsorption toxin tracking (SPATT) devices, toxin levels in particulate organic matter (POM, >1.5 μm) and whole water samples and cell concentrations from water samples as determined by microscopy and quantitative real-time PCR (qPCR). The dominant phycotoxin varied between SPATTs and all other phycotoxin sample types, and out of the 11 phycotoxins detected in oysters, only four and seven were detected in POM and whole water respectively, indicating phycotoxin profile mismatch between ecosystem compartments. Nevertheless, there were correlations between DA in oysters and whole water (simple linear regression [LR]: R2 = 0.6, p < 0.0001, n = 40), and PTX2 in oysters and SPATTs (LR: R2 = 0.3, p = 0.001, n = 36), providing additional monitoring tools for these phycotoxins, but oyster samples remain the best overall indicators of seafood safety.
Keywords: Azaspiracid; Domoic acid; Karlotoxin; Microcystin; Okadaic acid; Pectenotoxin.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Abbott B.C., Ballantine D. The toxin from Gymnodinium veneficum Ballantine. J. Mar. Biol. Assoc. U. K. 1957;36:169–189. doi: 10.1017/S0025315400017173. - DOI
-
- Anderson C.R., Sapiano M.R.P., Prasad M.B.K., Long W., Tango P.J., Brown C.W., Murtugudde R. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. Mar. Sys. 2010;83:127–140. doi: 10.1016/j.jmarsys.2010.04.003. - DOI
-
- Bachvaroff T.R., Adolf J.E., Squier A.H., Harvey H.R., Place A.R. Characterization and quantification of karlotoxins by liquid chromatography–mass spectrometry. Harmful Algae. 2008;7(4):473–484. doi: 10.1016/j.hal.2007.10.003. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

