Safety of Concurrent Radiation Therapy With Brentuximab Vedotin in the Treatment of Lymphoma
- PMID: 37448588
- PMCID: PMC10336411
- DOI: 10.1016/j.adro.2023.101279
Safety of Concurrent Radiation Therapy With Brentuximab Vedotin in the Treatment of Lymphoma
Abstract
Purpose: Purpose: Radiation therapy (RT) and the antibody-drug conjugate brentuximab vedotin (BV) are standard-of-care treatment options for patients with certain B and T-cell lymphomas; however, there are limited data exploring the safety of concurrent BV and RT (BVRT).
Methods and materials: We performed a single institutional retrospective review of 44 patients who received BVRT.
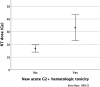
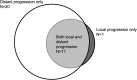
Results: Twenty percent of patients (9/44) developed new grade 2 or higher (G2+) hematologic toxicity (HT) after BVRT, which was associated with radiation dose (median dose of 35 Gy in those with new G2+ HT compared with 15 Gy in those without; P < .001). Acute G2+ elevation in aspartate transaminase or alanine transaminase level was associated with administration of concurrent chemotherapy with BVRT (57% vs 21%; P = .047) but was not associated with any RT factors. Local control (LC) was achieved in 24 of 42 patients (57%) with available follow-up. Ten patients (23%) proceeded to stem cell transplant or cellular therapy after BVRT at a median of 48 days (interquartile range, 27-188 days). At last follow-up, 10 patients (23%) remained without evidence of disease.
Conclusions: Our analysis demonstrates that the combination of BV and RT is well tolerated, though care should be taken during RT planning to reduce the risk of HT. This combination can be considered for patients in need of both local and systemic disease control.
© 2023 The Author(s).
Figures
References
-
- Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30–monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. - PubMed
-
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125:1394–1402. - PubMed
LinkOut - more resources
Full Text Sources

