Ropivacaine microsphere-loaded electroconductive nerve dressings for long-acting analgesia and functional recovery following diabetic peripheral nerve injury
- PMID: 37448664
- PMCID: PMC10336588
- DOI: 10.1016/j.mtbio.2023.100712
Ropivacaine microsphere-loaded electroconductive nerve dressings for long-acting analgesia and functional recovery following diabetic peripheral nerve injury
Abstract
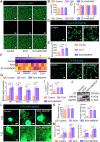
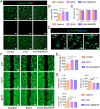
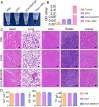
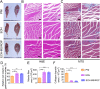
In recent years, electroconductive hydrogels (ECHs) have shown great potential in promoting nerve regeneration and motor function recovery following diabetic peripheral nerve injury (PNI), attributed to their similar electrical and mechanical characteristics to innate nervous tissue. It is well-established that PNI causes motor deficits and pain, especially in diabetics. Current evidence suggests that ropivacaine (ROP) encapsulated in poly lactic-co-glycolic acid (PLGA) microspheres (MSs) yield a sustained analgesic effect. In this study, an ECH electroconductive network loaded with MS/ROP (ECH-MS/ROP) was designed as a promising therapeutic approach for diabetic PNI to exert lasting analgesia and functional recovery. This dual delivery system allowed ROP's slow and sequential release, achieving sustained analgesia as demonstrated by our in vivo experiments. Meanwhile, this system was designed like a lamellar dressing, with desirable adhesive and self-curling properties, convenient for treating injured nerve tissues via automatically wrapping tube-like structures, facilitating the process of implantation. Our in vitro assays verified that ECH-MS/ROP was able to enhance the adhesion and motility of Schwann cells. Besides, both in vitro and in vivo studies substantiated that ECH-MS/ROP stimulated myelinated axon regeneration through the MEK/ERK signaling pathway, thereby improving muscular denervation atrophy and facilitating functional recovery. Therefore, this study suggests that the ECH-MS/ROP dressing provides a promising strategy for treating diabetic PNI to facilitate nerve regeneration, functional recovery and pain relief.
Keywords: Analgesia; Diabetic peripheral nerve injury; Electroconductive hydrogel; Nerve regeneration; Ropivacaine microspheres.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Li R., Li Y., Wu Y., Zhao Y., Chen H., Yuan Y., Xu K., Zhang H., Lu Y., Wang J., Li X., Jia X., Xiao J. Heparin-poloxamer thermosensitive hydrogel loaded with bFGF and NGF enhances peripheral nerve regeneration in diabetic rats. Biomaterials. 2018;168:24–37. doi: 10.1016/j.biomaterials.2018.03.044. - DOI - PMC - PubMed
-
- Lin T., Liu S., Chen S., Qiu S., Rao Z., Liu J., Zhu S., Yan L., Mao H., Zhu Q., Quan D., Liu X. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018;73:326–338. doi: 10.1016/j.actbio.2018.04.001. - DOI - PubMed
-
- Liu C., Fan L., Tian Z., Wen H., Zhou L., Guan P., Luo Y., Chan C., Tan G., Ning C., Rong L., Liu B. Self-curling electroconductive nerve dressing for enhancing peripheral nerve regeneration in diabetic rats. Bioact. Mater. 2021;6:3892–3903. doi: 10.1016/j.bioactmat.2021.03.034. - DOI - PMC - PubMed
-
- Cai Y., Huang Q., Wang P., Ye K., Zhao Z., Chen H., Liu Z., Liu H., Wong H., Tamtaji M., Zhang K., Xu F., Jin G., Zeng L., Xie J., Du Y., Hu Z., Sun D., Qin J., Lu X., Luo Z. Conductive hydrogel conduits with growth factor gradients for peripheral nerve repair in diabetics with non-suture tape. Adv. Healthc. Mater. 2022;11:1–12. doi: 10.1002/adhm.202200755. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

