Baricitinib retention rate: 'real-life' data from a mono-centric cohort of patients affected by rheumatoid arthritis
- PMID: 37448804
- PMCID: PMC10336222
- DOI: 10.3389/fmed.2023.1176613
Baricitinib retention rate: 'real-life' data from a mono-centric cohort of patients affected by rheumatoid arthritis
Abstract
Objectives: The aim of this retrospective study was to evaluate baricitinib retention rate in patients affected by rheumatoid arthritis. Secondary aims were to compare the impact on treatment persistence of monotherapy and other variables such as systemic corticosteroid use, line of treatment, disease duration, sex, biomarkers positivity, and Herpes Zoster virus infection.
Materials and methods: Patients with Rheumatoid Arthritis undergoing baricitinib were consecutively enrolled. Rheumatoid Arthritis diagnosis was performed with 2010 ACR/EULAR classification criteria. The cohort's demographic, clinical and therapeutical data were retrospectively collected. The whole follow-up duration was 104 weeks.
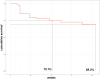
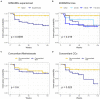
Results: Ninety-five patients affected by rheumatoid arthritis and treated with baricitinib were consecutively enrolled. At the end of follow-up, the overall retention rate was 69.3%. No statistically significant difference in retention rate was observed between patients treated with baricitinib in monotherapy or in combination with methotrexate (p = 0.638) while patients undergoing a steroidal treatment showed a significantly reduced treatment retention (p = 0.028). Contrarily, patients treated with baricitinib as a first-line b/tsDMARD showed higher drug retention (p = 0.002) compared to further treatment lines. Steroid employment, steroid dosage and previous treatment with bDMARDs correlated with risk of treatment discontinuation and at univariate analysis (p = 0.028, p < 0.001, and p = 0.002 respectively). Multivariate analysis confirmed significance for higher steroid dosage and previous treatment with bDMARDs (p = 0.002 and p = 0.046). No adverse events such as deep venous thrombosis, pulmonary embolism or tubercular infection/reactivation were reported during the study observation.
Conclusion: Our data show a good baricitinib retention rate after 12 and 24 months of observation (75.1 and 69.3%, respectively). In our cohort, concomitant treatment with methotrexate did not influence treatment persistence while retention was reduced in patients undergoing a steroidal treatment and/or in multi-failure subjects.
Keywords: JAK inhibitors; baricitinib; real-life; retention rate; rheumatoid arthritis; treatment.
Copyright © 2023 Baldi, Berlengiero, Falsetti, Cartocci, Conticini, D’Alessandro, D’Ignazio, Bardelli, Fabbroni, Cantarini, Frediani and Gentileschi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. (2010) 69:964–75. doi: 10.1136/ard.2009.126532, PMID: - DOI - PMC - PubMed
-
- Keystone EC, Taylor PC, Drescher E, Schlichting DE, Beattie SD, Berclaz PY, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis. (2015) 74:333–40. doi: 10.1136/annrheumdis-2014-206478, PMID: - DOI - PMC - PubMed
-
- Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. (2017) 69:506–17. doi: 10.1002/art.39953, PMID: - DOI - PMC - PubMed
-
- Schiff M, Takeuchi T, Fleischmann R, Gaich CL, DeLozier AM, Schlichting D, et al. Patient-reported outcomes of baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Res Ther. (2017) 19:208. doi: 10.1186/s13075-017-1410-1, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

