Polyimides as Promising Cathodes for Metal-Organic Batteries: A Comparison between Divalent (Ca2+, Mg2+) and Monovalent (Li+, Na+) Cations
- PMID: 37448980
- PMCID: PMC10336839
- DOI: 10.1021/acsaem.3c00969
Polyimides as Promising Cathodes for Metal-Organic Batteries: A Comparison between Divalent (Ca2+, Mg2+) and Monovalent (Li+, Na+) Cations
Abstract
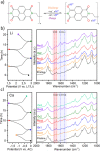
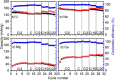
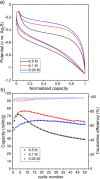
Ca- and Mg-based batteries represent a more sustainable alternative to Li-ion batteries. However, multivalent cation technologies suffer from poor cation mass transport. In addition, the development of positive electrodes enabling reversible charge storage currently represents one of the major challenges. Organic positive electrodes, in addition to being the most sustainable and potentially low-cost candidates, compared with their inorganic counterparts, currently present the best electrochemical performances in Ca and Mg cells. Unfortunately, organic positive electrodes suffer from relatively low capacity retention upon cycling, the origin of which is not yet fully understood. Here, 1,4,5,8-naphthalenetetracarboxylic dianhydride-derived polyimide was tested in Li, Na, Mg, and Ca cells for the sake of comparison in terms of redox potential, gravimetric capacities, capacity retention, and rate capability. The redox mechanisms were also investigated by means of operando IR experiments, and a parameter affecting most figures of merit has been identified: the presence of contact ion-pairs in the electrolyte.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Yang Y.; Okonkwo E. G.; Huang G.; Xu S.; Sun W.; He Y. On the sustainability of lithium ion battery industry – A review and perspective. Energy Storage Mater. 2021, 36, 186–212. 10.1016/j.ensm.2020.12.019. - DOI
-
- Gruber P. W.; Medina P. A.; Keoleian G. A.; Kesler S. E.; Everson M. P.; Wallington T. J. Global Lithium Availability. A Constraint for Electric Vehicles?. J. Ind. Ecol. 2011, 15, 760–775. 10.1111/j.1530-9290.2011.00359.x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
