In silico assessment of histotripsy-induced changes in catheter-directed thrombolytic delivery
- PMID: 37449013
- PMCID: PMC10336328
- DOI: 10.3389/fphys.2023.1225804
In silico assessment of histotripsy-induced changes in catheter-directed thrombolytic delivery
Abstract
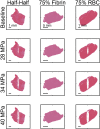
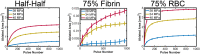
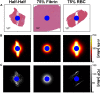
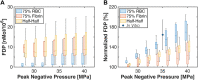
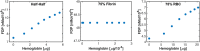
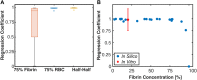
Introduction: For venous thrombosis patients, catheter-directed thrombolytic therapy is the standard-of-care to recanalize the occluded vessel. Limitations with thrombolytic drugs make the development of adjuvant treatments an active area of research. One potential adjuvant is histotripsy, a focused ultrasound therapy that lyses red blood cells within thrombus via the spontaneous generation of bubbles. Histotripsy has also been shown to improve the efficacy of thrombolytic drugs, though the precise mechanism of enhancement has not been elucidated. In this study, in silico calculations were performed to determine the contribution of histotripsy-induced changes in thrombus diffusivity to alter catheter-directed therapy. Methods: An established and validated Monte Carlo calculation was used to predict the extent of histotripsy bubble activity. The distribution of thrombolytic drug was computed with a finite-difference time domain (FDTD) solution of the perfusion-diffusion equation. The FDTD calculation included changes in thrombus diffusivity based on outcomes of the Monte Carlo calculation. Fibrin degradation was determined using the known reaction rate of thrombolytic drug. Results: In the absence of histotripsy, thrombolytic delivery was restricted in close proximity to the catheter. Thrombolytic perfused throughout the focal region for calculations that included the effects of histotripsy, resulting in an increased degree of fibrinolysis. Discussion: These results were consistent with the outcomes of in vitro studies, suggesting histotripsy-induced changes in the thrombus diffusivity are a primary mechanism for enhancement of thrombolytic drugs.
Keywords: Monte Carlo simulation; ablation; catheter-directed thrombolytics; diffusion; histotripsy; venous thrombosis.
Copyright © 2023 Bader, Flores Basterrechea and Hendley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abramowitz S. D., Kado H., Schor J., Annambhotla S., Mojibian H., Marino A. G., et al. (2023). Six-month deep vein thrombosis outcomes by chronicity: Analysis of the real-world ClotTriever outcomes registry. J. Vasc. Interv. Radiol. [Internet] 34 (5), 879–887.e4. e4. 10.1016/j.jvir.2022.12.480 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

