Recent advances in permeable polymersomes: fabrication, responsiveness, and applications
- PMID: 37449076
- PMCID: PMC10337762
- DOI: 10.1039/d3sc01707a
Recent advances in permeable polymersomes: fabrication, responsiveness, and applications
Abstract
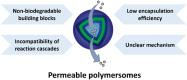
Polymersomes are vesicular nanostructures enclosed by a bilayer-membrane self-assembled from amphiphilic block copolymers, which exhibit higher stability compared with their biological analogues (e.g. liposomes). Due to their versatility, polymersomes have found various applications in different research fields such as drug delivery, nanomedicine, biological nanoreactors, and artificial cells. However, polymersomes prepared with high molecular weight components typically display low permeability to molecules and ions. It hence remains a major challenge to balance the opposing features of robustness and permeability of polymersomes. In this review, we focus on the design and strategies for fabricating permeable polymersomes, including polymersomes with intrinsic permeability, the formation of nanopores in the membrane bilayers by protein insertion, and the construction of stimuli-responsive polymersomes. Then, we highlight the applications of permeable polymersomes in the fields of biomimetic nanoreactors, artificial cells and organelles, and nanomedicine, to underline the challenges in the development of polymersomes as soft matter with biomedical utilities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures














Similar articles
-
Overcoming the Dilemma of Permeability and Stability of Polymersomes through Traceless Cross-Linking.Acc Chem Res. 2022 Dec 6;55(23):3404-3416. doi: 10.1021/acs.accounts.2c00442. Epub 2022 Nov 9. Acc Chem Res. 2022. PMID: 36351034
-
Stimuli-Responsive Polymersomes for Biomedical Applications.Biomacromolecules. 2017 Mar 13;18(3):649-673. doi: 10.1021/acs.biomac.6b01704. Epub 2017 Feb 17. Biomacromolecules. 2017. PMID: 28212005
-
Polymersomes as nanoreactors for preparative biocatalytic applications: current challenges and future perspectives.Bioprocess Biosyst Eng. 2018 Sep;41(9):1233-1246. doi: 10.1007/s00449-018-1953-9. Epub 2018 May 17. Bioprocess Biosyst Eng. 2018. PMID: 29777296 Review.
-
Role of Membrane Features on the Permeability Behavior of Polymersomes and the Potential Impacts on Drug Encapsulation and Release.Biomacromolecules. 2023 May 8;24(5):2291-2300. doi: 10.1021/acs.biomac.3c00162. Epub 2023 Apr 27. Biomacromolecules. 2023. PMID: 37103908
-
Stimuli-responsive polymersomes for cancer therapy.Biomater Sci. 2016 Jan;4(1):55-69. doi: 10.1039/c5bm00268k. Biomater Sci. 2016. PMID: 26456625 Review.
Cited by
-
Bioinspired photocatalytic systems towards compartmentalized artificial photosynthesis.Commun Chem. 2023 Dec 4;6(1):263. doi: 10.1038/s42004-023-01069-z. Commun Chem. 2023. PMID: 38049562 Free PMC article. Review.
-
Nanoenabling MbtI Inhibitors for Next-Generation Tuberculosis Therapy.J Med Chem. 2025 Mar 13;68(5):5312-5332. doi: 10.1021/acs.jmedchem.4c02386. Epub 2025 Mar 3. J Med Chem. 2025. PMID: 40029993 Free PMC article.
-
From Nano to Micro Polyion Complex Vesicles: Synthetic Cells with Membrane-Embedded Enzymes.ACS Appl Mater Interfaces. 2025 Aug 20;17(33):47426-47435. doi: 10.1021/acsami.5c11988. Epub 2025 Aug 10. ACS Appl Mater Interfaces. 2025. PMID: 40785247 Free PMC article.
-
Unlocking Intracellular Protein Delivery by Harnessing Polymersomes Synthesized at Microliter Volumes using Photo-PISA.Adv Mater. 2024 Dec;36(49):e2408000. doi: 10.1002/adma.202408000. Epub 2024 Oct 17. Adv Mater. 2024. PMID: 39417762 Free PMC article.
-
Unveiling the influence of oxygen on drug release dynamics in semipermeable polymersomes.Angew Chem Int Ed Engl. 2025 Feb 3;64(6):e202419087. doi: 10.1002/anie.202419087. Epub 2024 Nov 20. Angew Chem Int Ed Engl. 2025. PMID: 39446074 Free PMC article.
References
-
- Li H. Carter J. D. LaBean T. H. Mater. Today. 2009;12:24–32. doi: 10.1016/S1369-7021(09)70157-9. - DOI
Publication types
LinkOut - more resources
Full Text Sources

