Origin of Osteoclasts: Osteoclast Precursor Cells
- PMID: 37449346
- PMCID: PMC10346003
- DOI: 10.11005/jbm.2023.30.2.127
Origin of Osteoclasts: Osteoclast Precursor Cells
Abstract
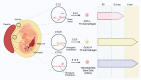
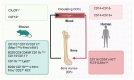
Osteoclasts are multinucleated bone-resorbing cells and a key player in bone remodeling for health and disease. Since the discovery of osteoclasts in 1873, the structure and function of osteoclasts and the molecular and cellular mechanisms of osteoclastogenesis have been extensively studied. Moreover, it has been well established that osteoclasts are differentiated in vitro from myeloid cells such as bone marrow macrophages or monocytes. The concept showing that osteoclasts are derived from a specific population (named osteoclast precursor cells [OCPs]) among myeloid cells has been long hypothesized. However, the specific precursor population of osteoclasts is not clearly defined yet. A growing body of work provides evidence of the developmental origin and lifespan of murine osteoclasts, particularly in vivo. Here, we review the emerging evidence that supports the existence of OCPs and discuss current insights into their identity.
Keywords: Osteoclast precursor cells; Osteoclast pregenitor; Osteoclasts.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
IL-1β differently stimulates proliferation and multinucleation of distinct mouse bone marrow osteoclast precursor subsets.J Leukoc Biol. 2016 Sep;100(3):513-23. doi: 10.1189/jlb.1A1215-543R. Epub 2016 Mar 8. J Leukoc Biol. 2016. PMID: 26957213
-
Increase in the Number of Bone Marrow Osteoclast Precursors at Different Skeletal Sites, Particularly in Long Bone and Jaw Marrow in Mice Lacking IL-1RA.Int J Mol Sci. 2020 May 27;21(11):3774. doi: 10.3390/ijms21113774. Int J Mol Sci. 2020. PMID: 32471111 Free PMC article.
-
Osteoclast fusion and regulation by RANKL-dependent and independent factors.World J Orthop. 2012 Dec 18;3(12):212-22. doi: 10.5312/wjo.v3.i12.212. World J Orthop. 2012. PMID: 23362465 Free PMC article.
-
The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell.J Cell Biochem. 2007 Dec 1;102(5):1130-9. doi: 10.1002/jcb.21553. J Cell Biochem. 2007. PMID: 17955494 Review.
-
Osteoclasts at Bone Remodeling: Order from Order.Results Probl Cell Differ. 2024;71:227-256. doi: 10.1007/978-3-031-37936-9_12. Results Probl Cell Differ. 2024. PMID: 37996681 Review.
Cited by
-
The effects of denosumab on osteoclast precursors in postmenopausal women: a possible explanation for the overshoot phenomenon after discontinuation.J Bone Miner Res. 2025 Mar 15;40(3):301-306. doi: 10.1093/jbmr/zjae170. J Bone Miner Res. 2025. PMID: 39418327 Free PMC article.
-
New Horizons: Translational Aspects of Osteomorphs.J Clin Endocrinol Metab. 2024 Apr 19;109(5):e1373-e1378. doi: 10.1210/clinem/dgad711. J Clin Endocrinol Metab. 2024. PMID: 38060842 Free PMC article. Review.
-
New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients.Int J Mol Sci. 2024 Jan 30;25(3):1710. doi: 10.3390/ijms25031710. Int J Mol Sci. 2024. PMID: 38338988 Free PMC article.
-
(D-Ala2)GIP Inhibits Inflammatory Bone Resorption by Suppressing TNF-α and RANKL Expression and Directly Impeding Osteoclast Formation.Int J Mol Sci. 2024 Feb 22;25(5):2555. doi: 10.3390/ijms25052555. Int J Mol Sci. 2024. PMID: 38473802 Free PMC article.
-
Chronic alcohol consumption enhances the differentiation capacity of hematopoietic stem and progenitor cells into osteoclast precursors.bioRxiv [Preprint]. 2025 Feb 8:2025.02.05.636743. doi: 10.1101/2025.02.05.636743. bioRxiv. 2025. Update in: Am J Pathol. 2025 Jul 17:S0002-9440(25)00244-5. doi: 10.1016/j.ajpath.2025.06.010. PMID: 39975302 Free PMC article. Updated. Preprint.

