Optimal place of treatment for young infants aged less than two months with any low-mortality-risk sign of possible serious bacterial infection: Study Protocol for a randomised controlled trial from low- and middle-income countries
- PMID: 37449353
- PMCID: PMC10346131
- DOI: 10.7189/jogh.13.04055
Optimal place of treatment for young infants aged less than two months with any low-mortality-risk sign of possible serious bacterial infection: Study Protocol for a randomised controlled trial from low- and middle-income countries
Abstract
Background: World Health Organization (WHO) recommends hospitalisation and injectable antibiotics for clinical sepsis / possible serious bacterial infection (PSBI) in young infants up to two months of age. However, some young infants with low-mortality risk signs of PSBI may not require hospitalisation, for which evidence needs to be generated.
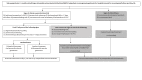
Methods: This is a protocol for a multicentre, individually randomised, open-label trial that will be conducted in seven sites in six countries Bangladesh, Ethiopia, India (two sites), Nigeria, Pakistan and Tanzania. All sites will use this common protocol with the same study design, inclusion of participants, intervention, comparison, and outcomes, as well as quality control and analysis procedures to contribute to the overall sample size. All young infants (age <60 days) presenting at study hospitals with any single low-mortality risk sign (high body temperature ≥38°C, severe chest indrawing, or fast breathing of ≥60 breaths per minute in <7 days old infants) will be randomised to either outpatient care with injectable gentamicin for two days and oral amoxicillin for seven days (intervention) or inpatient care with injection gentamicin plus injection ampicillin along with supportive treatment, where needed, for seven days (control). We plan to enrol 7000 eligible young infants, 3500 infants in each of the two study arms. A trained and standardised independent outcome assessor will visit all enrolled cases on days two, four, eight and 15 post-randomisation to assess the study outcomes in both intervention and control groups. The primary outcome of poor clinical outcome, defined as death within two weeks of initiation of treatment, deterioration during the 7-day treatment period, or persistence of the presenting sign at the end of the 7-day treatment period, will be compared to assess if the outpatient treatment leads to superior or at least non-inferior clinical outcome than inpatient treatment. The selected sites have extensive research experience. The methods and all study procedures will be harmonised through central training of research staff by WHO, standardisation exercises for clinical signs, central data coordination centre and internal and external monitoring. Continuous evaluation of the enrolment by the sites will be carried out through regular calls, databased monitoring, and site visits by WHO monitors. This trial has received ethical approvals from the WHO and local site institutional ethics committees.
Discussion: If the results show that young infants with any single low-mortality risk PSBI sign can be effectively and safely treated on an outpatient basis, it may substantially increase access to treatment for infants and families with poor access to health facilities. It may also reduce the human, financial and material costs to the health system and allow the currently overloaded health facilities to focus on more critically ill infants. This evidence will contribute toward making a case for reviewing the current WHO PSBI management guideline.
Registration: International Standard Randomised Controlled Trial Number ISRCTN44033252.
Copyright © 2023 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Figures
References
-
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels and Trends in Child Mortality: Report 2022. New York: United Nations Children’s Fund; 2022. Available: https://data.unicef.org/resources/levels-and-trends-in-child-mortality/. Accessed 8 February 2023.
-
- Alliance for Maternal and Newborn Health Improvement (AMANHI) mortality study group Population-based rates, timing, and causes of maternal deaths, stillbirths, and neonatal deaths in south Asia and sub-Saharan Africa: a multi-country prospective cohort study. Lancet Glob Health. 2018;6:e1297-308. 10.1016/S2214-109X(18)30385-1 - DOI - PMC - PubMed
-
- World Health Organization. Integrated Management of Childhood Illness: management of the sick young infant aged up to 2 months. IMCI chart booklet. Geneva, Switzerland: WHO; 2019. Available: https://www.who.int/publications/i/item/9789241516365. Accessed 8 February 2023.
-
- World Health Organization. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2nd ed. Switzerland: World Health Organization; 2013. Available at: https://apps.who.int/iris/bitstream/handle/10665/81170/9789241548373_eng.... Accessed 8 February 2023. - PubMed
-
- World Health Organization. Guideline: Managing possible serious bacterial infection in young infants when referral is not feasible. Switzerland: WHO; 2015. Available: https://www.who.int/publications/i/item/9789241509268. Accessed 8 February 2023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
