Nitrification in acidic and alkaline environments
- PMID: 37449414
- PMCID: PMC10427799
- DOI: 10.1042/EBC20220194
Nitrification in acidic and alkaline environments
Abstract
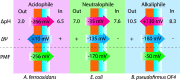
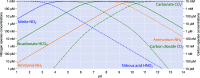
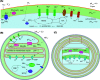
Aerobic nitrification is a key process in the global nitrogen cycle mediated by microorganisms. While nitrification has primarily been studied in near-neutral environments, this process occurs at a wide range of pH values, spanning ecosystems from acidic soils to soda lakes. Aerobic nitrification primarily occurs through the activities of ammonia-oxidising bacteria and archaea, nitrite-oxidising bacteria, and complete ammonia-oxidising (comammox) bacteria adapted to these environments. Here, we review the literature and identify knowledge gaps on the metabolic diversity, ecological distribution, and physiological adaptations of nitrifying microorganisms in acidic and alkaline environments. We emphasise that nitrifying microorganisms depend on a suite of physiological adaptations to maintain pH homeostasis, acquire energy and carbon sources, detoxify reactive nitrogen species, and generate a membrane potential at pH extremes. We also recognize the broader implications of their activities primarily in acidic environments, with a focus on agricultural productivity and nitrous oxide emissions, as well as promising applications in treating municipal wastewater.
Keywords: archaea; metabolism; nitrification.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
-
- Ward B.B. (2013) Nitrification. In Encyclopedia of Ecology Second Edition(Fath B., ed.), pp. 351–358, Elsevier, Oxford: 10.1016/B978-0-12-409548-9.00697-7 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

