Mechanisms of bioleaching: iron and sulfur oxidation by acidophilic microorganisms
- PMID: 37449416
- PMCID: PMC10427800
- DOI: 10.1042/EBC20220257
Mechanisms of bioleaching: iron and sulfur oxidation by acidophilic microorganisms
Abstract
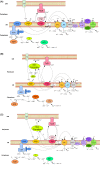
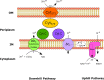
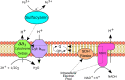
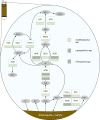
Bioleaching offers a low-input method of extracting valuable metals from sulfide minerals, which works by exploiting the sulfur and iron metabolisms of microorganisms to break down the ore. Bioleaching microbes generate energy by oxidising iron and/or sulfur, consequently generating oxidants that attack sulfide mineral surfaces, releasing target metals. As sulfuric acid is generated during the process, bioleaching organisms are typically acidophiles, and indeed the technique is based on natural processes that occur at acid mine drainage sites. While the overall concept of bioleaching appears straightforward, a series of enzymes is required to mediate the complex sulfur oxidation process. This review explores the mechanisms underlying bioleaching, summarising current knowledge on the enzymes driving microbial sulfur and iron oxidation in acidophiles. Up-to-date models are provided of the two mineral-defined pathways of sulfide mineral bioleaching: the thiosulfate and the polysulfide pathway.
Keywords: Acidophiles; Bioleaching; Iron oxidation; Sulfur oxidation.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




Similar articles
-
Progress in bioleaching: fundamentals and mechanisms of microbial metal sulfide oxidation - part A.Appl Microbiol Biotechnol. 2022 Nov;106(21):6933-6952. doi: 10.1007/s00253-022-12168-7. Epub 2022 Oct 4. Appl Microbiol Biotechnol. 2022. PMID: 36194263 Free PMC article. Review.
-
Life in heaps: a review of microbial responses to variable acidity in sulfide mineral bioleaching heaps for metal extraction.Res Microbiol. 2016 Sep;167(7):576-86. doi: 10.1016/j.resmic.2016.05.007. Epub 2016 Jun 6. Res Microbiol. 2016. PMID: 27283362 Review.
-
Presentation on mechanisms and applications of chalcopyrite and pyrite bioleaching in biohydrometallurgy - a presentation.Biotechnol Rep (Amst). 2014 Sep 16;4:107-119. doi: 10.1016/j.btre.2014.09.003. eCollection 2014 Dec. Biotechnol Rep (Amst). 2014. PMID: 28626669 Free PMC article. Review.
-
Competitive Growth of Sulfate-Reducing Bacteria with Bioleaching Acidophiles for Bioremediation of Heap Bioleaching Residue.Int J Environ Res Public Health. 2020 Apr 15;17(8):2715. doi: 10.3390/ijerph17082715. Int J Environ Res Public Health. 2020. PMID: 32326522 Free PMC article.
-
Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation--part A.Appl Microbiol Biotechnol. 2013 Sep;97(17):7529-41. doi: 10.1007/s00253-013-4954-2. Epub 2013 May 30. Appl Microbiol Biotechnol. 2013. PMID: 23720034 Review.
Cited by
-
From Genes to Bioleaching: Unraveling Sulfur Metabolism in Acidithiobacillus Genus.Genes (Basel). 2023 Sep 8;14(9):1772. doi: 10.3390/genes14091772. Genes (Basel). 2023. PMID: 37761912 Free PMC article. Review.
-
Adaptive response of the holdase chaperone network of Acidithiobacillus ferrooxidans ATCC 23270 to stresses and energy sources.World J Microbiol Biotechnol. 2025 Apr 1;41(4):121. doi: 10.1007/s11274-025-04325-7. World J Microbiol Biotechnol. 2025. PMID: 40167894
-
Pyrite stimulates the growth and sulfur oxidation capacity of anoxygenic phototrophic sulfur bacteria in euxinic environments.Sci Adv. 2025 Apr 18;11(16):eadu7080. doi: 10.1126/sciadv.adu7080. Epub 2025 Apr 18. Sci Adv. 2025. PMID: 40249799 Free PMC article.
-
Isolation, Sphalerite Bioleaching, and Whole Genome Sequencing of Acidithiobacillus ferriphilus QBS3 from Zinc-Rich Sulfide Mine Drainage.Life (Basel). 2025 May 15;15(5):792. doi: 10.3390/life15050792. Life (Basel). 2025. PMID: 40430218 Free PMC article.
-
Overexpression of sulfide:quinone reductase (SQR) in Acidithiobacillus ferrooxidans enhances sulfur, pyrite, and pyrrhotite oxidation.Appl Environ Microbiol. 2025 Apr 23;91(4):e0017025. doi: 10.1128/aem.00170-25. Epub 2025 Mar 25. Appl Environ Microbiol. 2025. PMID: 40130842 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

