Targeting Neutrophil α9 Improves Functional Outcomes After Stroke in Mice With Obesity-Induced Hyperglycemia
- PMID: 37449422
- PMCID: PMC10529694
- DOI: 10.1161/STROKEAHA.123.042714
Targeting Neutrophil α9 Improves Functional Outcomes After Stroke in Mice With Obesity-Induced Hyperglycemia
Abstract
Background: Obesity-induced hyperglycemia is a significant risk factor for stroke. Integrin α9β1 is expressed on neutrophils and stabilizes adhesion to the endothelium via ligands, including Fn-EDA (fibronectin containing extra domain A) and tenascin C. Although myeloid deletion of α9 reduces susceptibility to ischemic stroke, it is unclear whether this is mediated by neutrophil-derived α9. We determined the role of neutrophil-specific α9 in stroke outcomes in a mice model with obesity-induced hyperglycemia.
Methods: α9Neu-KO (α9fl/flMRP8Cre+) and littermate control α9WT (α9fl/flMRP8Cre-) mice were fed on a 60% high-fat diet for 20 weeks to induce obesity-induced hyperglycemia. Functional outcomes were evaluated up to 28 days after stroke onset in mice of both sexes using a transient (30 minutes) middle cerebral artery ischemia. Infarct volume (magnetic resonance imaging) and postreperfusion thrombo-inflammation (thrombi, fibrin, neutrophil, phospho-nuclear factor kappa B [p-NFκB], TNF [tumor necrosis factor]-α, and IL [interleukin]-1β levels, markers of neutrophil extracellular traps) were measured post 6 or 48 hours of reperfusion. In addition, functional outcomes (modified Neurological Severity Score, rota-rod, corner, and wire-hanging test) were measured for up to 4 weeks.
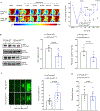
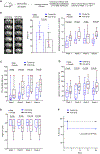
Results: Stroke upregulated neutrophil α9 expression more in obese mice (P<0.05 versus lean mice). Irrespective of sex, deletion of neutrophil α9 improved functional outcomes up to 4 weeks, concomitant with reduced infarct, improved cerebral blood flow, decreased postreperfusion thrombo-inflammation, and neutrophil extracellular traps formation (NETosis) (P<0.05 versus α9WT obese mice). Obese α9Neu-KO mice were less susceptible to thrombosis in FeCl3 injury-induced carotid thrombosis model. Mechanistically, we found that α9/cellular fibronectin axis contributes to NETosis via ERK (extracellular signal-regulated kinase) and PAD4 (peptidyl arginine deiminase 4), and neutrophil α9 worsens stroke outcomes via cellular fibronectin-EDA but not tenascin C. Obese wild-type mice infused with anti-integrin α9 exhibited improved functional outcomes up to 4 weeks (P<0.05 versus vehicle).
Conclusions: Genetic ablation of neutrophil-specific α9 or pharmacological inhibition improves long-term functional outcomes after stroke in mice with obesity-induced hyperglycemia, most likely by limiting thrombo-inflammation.
Keywords: hyperglycemia; ischemic stroke; mice; neutrophils; obesity; risk factor.
Conflict of interest statement
Figures




References
-
- Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

