The importance of laminin at the blood-brain barrier
- PMID: 37449589
- PMCID: PMC10358660
- DOI: 10.4103/1673-5374.373677
The importance of laminin at the blood-brain barrier
Abstract
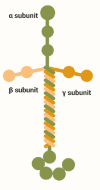
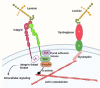
The blood-brain barrier is a unique property of central nervous system blood vessels that protects sensitive central nervous system cells from potentially harmful blood components. The mechanistic basis of this barrier is found at multiple levels, including the adherens and tight junction proteins that tightly bind adjacent endothelial cells and the influence of neighboring pericytes, microglia, and astrocyte endfeet. In addition, extracellular matrix components of the vascular basement membrane play a critical role in establishing and maintaining blood-brain barrier integrity, not only by providing an adhesive substrate for blood-brain barrier cells to adhere to, but also by providing guidance cues that strongly influence vascular cell behavior. The extracellular matrix protein laminin is one of the most abundant components of the basement membrane, and several lines of evidence suggest that it plays a key role in directing blood-brain barrier behavior. In this review, we describe the basic structure of laminin and its receptors, the expression patterns of these molecules in central nervous system blood vessels and how they are altered in disease states, and most importantly, how genetic deletion of different laminin isoforms or their receptors reveals the contribution of these molecules to blood-brain barrier function and integrity. Finally, we discuss some of the important unanswered questions in the field and provide a "to-do" list of some of the critical outstanding experiments.
Keywords: astrocytes; basement membrane; blood vessels; blood-brain barrier integrity; dystroglycan; endothelial cells; inflammation; integrins; laminin; pericytes.
Conflict of interest statement
None
Figures

References
-
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR, del Zoppo GJ. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:1038–1050. - PubMed
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

