Sacubitril/valsartan for cardioprotection in breast cancer (MAINSTREAM): design and rationale of the randomized trial
- PMID: 37449716
- PMCID: PMC10567668
- DOI: 10.1002/ehf2.14466
Sacubitril/valsartan for cardioprotection in breast cancer (MAINSTREAM): design and rationale of the randomized trial
Abstract
Aims: In recent years, survival in patients with breast cancer has increased. Despite the improvement in outcomes of those patients, the risk of treatment-related cardiotoxicity remains high, and its presence has been associated with a higher risk of treatment termination and thus lower therapeutic efficacy. Prior trials demonstrated that a preventive initiation of heart failure drugs, including the renin-angiotensin-aldosterone inhibitors, might reduce the risk of treatment-related cardiotoxicity. However, to date, no study investigated the efficacy of sacubitril/valsartan, a novel antineurohormonal drug shown to be superior to the previous therapies, in the prevention of cardiotoxicity in patients with early-stage breast cancer, which is the aim of the trial.
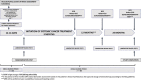
Methods and results: MAINSTREAM is a randomized, placebo-controlled, double-blind, multicentre, clinical trial. After the run-in period, a total of 480 patients with early breast cancer undergoing treatment with anthracyclines and/or anti-human epidermal growth factor receptor 2 drugs will be randomized to the highest tolerated dose of sacubitril/valsartan, being preferably 97/103 mg twice daily or placebo in 1:1 ratio. The patients will be monitored, including routine transthoracic echocardiography (TTE) and laboratory biomarker monitoring, for 24 months. The primary endpoint of the trial will be the occurrence of a decrease in left ventricular ejection fraction by ≥5% in TTE within 24 months. The key secondary endpoints will be the composite endpoint of death from any cause or hospitalization for heart failure, as well as other imaging, laboratory, and clinical outcomes, including the occurrence of the cancer therapy-related cardiac dysfunction resulting in the necessity to initiate treatment. The first patients are expected to be recruited in the coming months, and the estimated completion of the study and publication of the results are expected in December 2027, pending recruitment.
Conclusions: The MAINSTREAM trial will determine the efficacy and safety of treatment with sacubitril/valsartan as a prevention of cardiotoxicity in patients with early breast cancer (ClinicalTrials.gov number: NCT05465031).
Keywords: Breast cancer; Cardio-oncology; Cardiotoxicity; Heart failure; Randomized trial; Sacubitril/valsartan.
© 2023 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
None declared.
Figures
References
-
- Breast cancer statistics [Internet]. https://www.wcrf.org/cancer‐trends/breast‐cancer‐statistics/. Accessed 07 June 2023.
-
- Lyon AR, López‐Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler‐Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH, ESC Scientific Document Group . 2022 ESC guidelines on cardio‐oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio‐Oncology Society (IC‐OS). Eur Heart J [Internet]. 2022; 43: 4229–4361. http://www.ncbi.nlm.nih.gov/pubmed/36017568. Accessed 07 June 2023. - PubMed
-
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation [Internet]. 2015; 131: 1981–1988. http://www.ncbi.nlm.nih.gov/pubmed/25948538. Accessed 07 June 2023. - PubMed
-
- Mohan N, Jiang J, Dokmanovic M, Wu WJ. Trastuzumab‐mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther [Internet]. 2018; 1: 13–17. http://www.ncbi.nlm.nih.gov/pubmed/30215054. Accessed 07 June 2023. - PMC - PubMed
-
- Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy‐induced cardiotoxicity. Trends Cardiovasc Med [Internet]. 2020; 30: 22–28. http://www.ncbi.nlm.nih.gov/pubmed/30745071. Accessed 07 June 2023. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials