"Double-edged sword" effect of reactive oxygen species (ROS) in tumor development and carcinogenesis
- PMID: 37449744
- PMCID: PMC10669002
- DOI: 10.33549/physiolres.935007
"Double-edged sword" effect of reactive oxygen species (ROS) in tumor development and carcinogenesis
Abstract
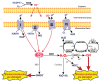
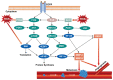
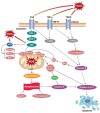
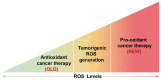
Reactive oxygen species (ROS) are small reactive molecules produced by cellular metabolism and regulate various physiological and pathological functions. Many studies have shown that ROS plays an essential role in the proliferation and inhibition of tumor cells. Different concentrations of ROS can have a "double-edged sword" effect on the occurrence and development of tumors. A certain concentration of ROS can activate growth-promoting signals, enhance the proliferation and invasion of tumor cells, and cause damage to biomacromolecules such as proteins and nucleic acids. However, ROS can enhance the body's antitumor signal at higher levels by initiating oxidative stress-induced apoptosis and autophagy in tumor cells. This review analyzes ROS's unique bidirectional regulation mechanism on tumor cells, focusing on the key signaling pathways and regulatory factors that ROS affect the occurrence and development of tumors and providing ideas for an in-depth understanding of the mechanism of ROS action and its clinical application.
Conflict of interest statement
There is no conflict of interest.
Figures
References
-
- Rincheval V, Bergeaud M, Mathieu L, Leroy J, Guillaume A, Mignotte B, Le Floch N, Vayssière JL. Differential effects of Bcl-2 and caspases on mitochondrial permeabilization during endogenous or exogenous reactive oxygen species-induced cell death: a comparative study of H2O2, paraquat t-BHP, etoposide, TNF-α-induced cell death. Cell Biol Toxicol. 2012;28:239–253. doi: 10.1007/s10565-012-9219-9. - DOI - PubMed
