Site-Specific Glycation of Human Heat Shock Protein (Hsp27) Enhances Its Chaperone Activity
- PMID: 37449780
- PMCID: PMC10442856
- DOI: 10.1021/acschembio.3c00214
Site-Specific Glycation of Human Heat Shock Protein (Hsp27) Enhances Its Chaperone Activity
Abstract
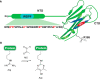
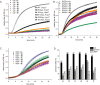
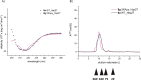
Non-enzymatic posttranslational modifications are believed to affect at least 30% of human proteins, commonly termed glycation. Many of these modifications are implicated in various pathological conditions, e.g., cataract, diabetes, neurodegenerative diseases, and cancer. Chemical protein synthesis enables access to full-length proteins carrying site-specific modifications. One such modification, argpyrimidine (Apy), has been detected in human small heat shock protein Hsp27 and closely related proteins in patient-derived tissues. Thus far, studies have looked into only artificial mixtures of Apy modifications, and only one has analyzed Apy188. We were interested in understanding the impact of such individual Apy modifications on five different arginine sites within the crucial N-terminal domain of Hsp27. By combining protein semisynthesis with biochemical assays on semisynthetic Hsp27 analogues with single-point Apy modification at those sites, we have shown how a seemingly minimal modification within this region results in dramatically altered functional attributes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Impaired Chaperone Activity of Human Heat Shock Protein Hsp27 Site-Specifically Modified with Argpyrimidine.Angew Chem Int Ed Engl. 2016 Sep 12;55(38):11397-402. doi: 10.1002/anie.201605366. Epub 2016 Jul 21. Angew Chem Int Ed Engl. 2016. PMID: 27440458
-
Methylglyoxal modifies heat shock protein 27 in glomerular mesangial cells.FEBS Lett. 2003 Sep 11;551(1-3):113-8. doi: 10.1016/s0014-5793(03)00874-3. FEBS Lett. 2003. PMID: 12965214
-
Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27.J Cell Biochem. 2006 Sep 1;99(1):279-91. doi: 10.1002/jcb.20781. J Cell Biochem. 2006. PMID: 16615138
-
Cataract as a conformational disease--the Maillard reaction, alpha-crystallin and chemotherapy.Cell Mol Biol (Noisy-le-grand). 1998 Nov;44(7):1047-50. Cell Mol Biol (Noisy-le-grand). 1998. PMID: 9846886 Review.
-
Methylglyoxal and Small Heat Shock Proteins.Biochemistry (Mosc). 2017 Jul;82(7):751-759. doi: 10.1134/S000629791707001X. Biochemistry (Mosc). 2017. PMID: 28918740 Review.
Cited by
-
Potential for targeting small heat shock protein modifications.Trends Pharmacol Sci. 2024 Jul;45(7):583-585. doi: 10.1016/j.tips.2024.04.002. Epub 2024 May 3. Trends Pharmacol Sci. 2024. PMID: 38704305 Free PMC article.
-
O-GlcNAc Modification Alters the Chaperone Activity of HSP27 Charcot-Marie-Tooth Type 2 (CMT2) Variants in a Mutation-Selective Fashion.ACS Chem Biol. 2023 Aug 18;18(8):1705-1712. doi: 10.1021/acschembio.3c00292. Epub 2023 Aug 4. ACS Chem Biol. 2023. PMID: 37540114 Free PMC article.
-
Advances in the chemical synthesis of human proteoforms.Sci China Life Sci. 2025 Sep;68(9):2515-2549. doi: 10.1007/s11427-024-2860-5. Epub 2025 Apr 8. Sci China Life Sci. 2025. PMID: 40210795 Review.
-
An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition.Biophys Rev. 2024 Apr 12;16(2):189-218. doi: 10.1007/s12551-024-01188-4. eCollection 2024 Apr. Biophys Rev. 2024. PMID: 38737201 Free PMC article. Review.
-
Non-enzymatic posttranslational protein modifications in protein aggregation and neurodegenerative diseases.RSC Chem Biol. 2024 Dec 19;6(2):129-149. doi: 10.1039/d4cb00221k. eCollection 2025 Feb 5. RSC Chem Biol. 2024. PMID: 39722676 Free PMC article. Review.
References
-
- Walsh C.Posttranslational modification of proteins: expanding nature’s inventory; Roberts and Company Publishers, 2006.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

