Site-Specific Glycation of Human Heat Shock Protein (Hsp27) Enhances Its Chaperone Activity
- PMID: 37449780
- PMCID: PMC10442856
- DOI: 10.1021/acschembio.3c00214
Site-Specific Glycation of Human Heat Shock Protein (Hsp27) Enhances Its Chaperone Activity
Abstract
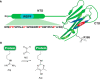
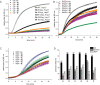
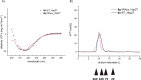
Non-enzymatic posttranslational modifications are believed to affect at least 30% of human proteins, commonly termed glycation. Many of these modifications are implicated in various pathological conditions, e.g., cataract, diabetes, neurodegenerative diseases, and cancer. Chemical protein synthesis enables access to full-length proteins carrying site-specific modifications. One such modification, argpyrimidine (Apy), has been detected in human small heat shock protein Hsp27 and closely related proteins in patient-derived tissues. Thus far, studies have looked into only artificial mixtures of Apy modifications, and only one has analyzed Apy188. We were interested in understanding the impact of such individual Apy modifications on five different arginine sites within the crucial N-terminal domain of Hsp27. By combining protein semisynthesis with biochemical assays on semisynthetic Hsp27 analogues with single-point Apy modification at those sites, we have shown how a seemingly minimal modification within this region results in dramatically altered functional attributes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Walsh C.Posttranslational modification of proteins: expanding nature’s inventory; Roberts and Company Publishers, 2006.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

