The COMPARE head-to-head, randomized controlled trial of SEL-212 (pegadricase plus rapamycin-containing nanoparticle, ImmTOR™) versus pegloticase for refractory gout
- PMID: 37449908
- PMCID: PMC10986798
- DOI: 10.1093/rheumatology/kead333
The COMPARE head-to-head, randomized controlled trial of SEL-212 (pegadricase plus rapamycin-containing nanoparticle, ImmTOR™) versus pegloticase for refractory gout
Abstract
Objectives: Serum urate (SU) lowering with PEGylated uricases in gout can reduce flares and tophi. However, treatment-emergent anti-drug antibodies adversely affect safety and efficacy and the currently approved PEGylated uricase pegloticase requires twice-monthly infusions. Investigational SEL-212 therapy aims to promote uricase-specific tolerance via monthly sequential infusions of a proprietary rapamycin-containing nanoparticle (ImmTOR) and pegadricase.
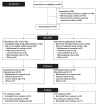
Methods: COMPARE was a randomized, phase 2, open-label trial of SEL-212 vs pegloticase in adults with refractory gout. SEL-212 [ImmTOR (0.15 mg/kg) and pegadricase (0.2 mg/kg)] was infused monthly or pegloticase (8 mg) twice monthly for 6 months. The primary endpoint was the proportion of participants with SU <6 mg/dl for ≥80% of the time during 3 and 6 months. Secondary outcomes were mean SU, gout flares, number of tender and/or swollen joints and safety.
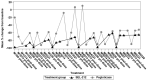
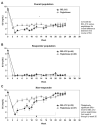
Results: During months 3 and 6 combined, numerically more participants achieved and maintained a SU <6 mg/dl for ≥80% of the time with SEL-212 vs pegloticase (53.0% vs 46.0%, P = 0.181). The percentage reductions in SU levels were statistically greater during months 3 and 6 with SEL-212 vs pegloticase (-73.79% and -47.96%, P = 0.0161). Reductions in gout flare incidence and number of tender and/or swollen joints were comparable between treatments. There were numerical differences between the most common treatment-related adverse events of interest with SEL-212 and pegloticase: gout flares (60.2% vs 50.6%), infections (25.3% vs 18.4%) and infusion-related reactions (15.7% vs 11.5%), respectively. Stomatitis (and related terms) was experienced by eight participants (9.6%) with SEL-212 and none with pegloticase. Stomatitis, a known event for rapamycin, was associated with ImmTOR only.
Conclusions: SEL-212 efficacy and tolerability were comparable to pegloticase in refractory gout. This was associated with a substantial reduction in treatment burden with SEL-212 due to decreased infusion frequency vs pegloticase.
Clinical trial registration: NCT03905512.
Keywords: SEL-212; gout; nanotechnology; pegadricase; pegloticase; rapamycin; serum urate; uricase.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Ruoff G, Edwards NL.. Overview of serum uric acid treatment targets in gout: why less than 6 mg/dL? Postgrad Med 2016;128:706–15. - PubMed
-
- Schlesinger N, Lipsky PE.. Pegloticase treatment of chronic refractory gout: update on efficacy and safety. Semin Arthritis Rheum 2020;50(3 Suppl):S31–8. - PubMed
-
- Fels E, Sundy JS.. Refractory gout: what is it and what to do about it? Curr Opin Rheumatol 2008;20:198–202. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical