Gaze patterns and brain activations in humans and marmosets in the Frith-Happé theory-of-mind animation task
- PMID: 37449983
- PMCID: PMC10435231
- DOI: 10.7554/eLife.86327
Gaze patterns and brain activations in humans and marmosets in the Frith-Happé theory-of-mind animation task
Abstract
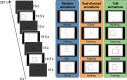
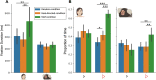
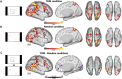
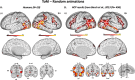
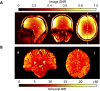
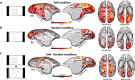
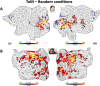
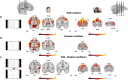
Theory of Mind (ToM) refers to the cognitive ability to attribute mental states to other individuals. This ability extends even to the attribution of mental states to animations featuring simple geometric shapes, such as the Frith-Happé animations in which two triangles move either purposelessly (Random condition), exhibit purely physical movement (Goal-directed condition), or move as if one triangle is reacting to the other triangle's mental states (ToM condition). While this capacity in humans has been thoroughly established, research on nonhuman primates has yielded inconsistent results. This study explored how marmosets (Callithrix jacchus), a highly social primate species, process Frith-Happé animations by examining gaze patterns and brain activations of marmosets and humans as they observed these animations. We revealed that both marmosets and humans exhibited longer fixations on one of the triangles in ToM animations, compared to other conditions. However, we did not observe the same pattern of longer overall fixation duration on the ToM animations in marmosets as identified in humans. Furthermore, our findings reveal that both species activated extensive and comparable brain networks when viewing ToM versus Random animations, suggesting that marmosets differentiate between these scenarios similarly to humans. While marmosets did not mimic human overall fixation patterns, their gaze behavior and neural activations indicate a distinction between ToM and non-ToM scenarios. This study expands our understanding of nonhuman primate cognitive abilities, shedding light on potential similarities and differences in ToM processing between marmosets and humans.
Keywords: Callithrix jacchus; Frith-Happé animations; Theory of Mind; eye tracking; fMRI; human; marmoset monkeys; neuroscience.
Plain language summary
In our daily life, we often guess what other people are thinking or intending to do, based on their actions. This ability to ascribe thoughts, intentions or feelings to others is known as Theory of Mind. While we often use our Theory of Mind to understand other humans and interpret social interactions, we can also apply our Theory of Mind to assign feelings and thoughts to animals and even inanimate objects. For example, people watching a movie where the characters are represented by simple shapes, such as triangles, can still see a story unfold, because they infer the triangles’ intentions based on what they see on the screen. While it is clear that humans have a Theory of Mind, how the brain manages this capacity and whether other species have similar abilities remain open questions. Dureux et al. used animations showing abstract shapes engaging in social interactions and advanced brain imaging techniques to compare how humans and marmosets – a type of monkey that is very social and engages in shared childcare – interpret social cues. By comparing the eye movements and brain activity of marmosets to human responses, Dureux et al. wanted to uncover common strategies used by both species to understand social signals, and gain insight into how these strategies have evolved. Dureux et al. found that, like humans, marmosets seem to perceive a difference between shapes interacting socially and moving randomly. Not only did their gaze linger longer on certain shapes in the social scenario, but their brain activity also mirrored that of humans viewing the same scenes. This suggests that, like humans, marmosets possess an inherent ability to interpret social scenarios, even when they are presented in an abstract form, providing a fresh perspective on primates’ abilities to interpret social cues. The findings of Dureux et al. have broad implications for our understanding of human social behavior and could lead to the development of better communication strategies, especially for individuals social cognitive conditions, such as Autism Spectrum Disorder. However, further research will be needed to understand the neural processes underpinning the interpretation of social interactions. Dureux et al.’s research indicates that the marmoset monkey may be the ideal organism to perform this research on.
© 2023, Dureux et al.
Conflict of interest statement
AD, AZ, JS, RM, SE No competing interests declared
Figures
Update of
References
-
- Abell F, Happé F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Development. 2000;15:1–16. doi: 10.1016/S0885-2014(00)00014-9. - DOI
-
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, Hartley T, Footer O, Bjork JM, Poldrack R, Smith S, Johansen-Berg H, Snyder AZ, Van Essen DC, WU-Minn HCP Consortium Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

