Fast-Acting Insulin Aspart in Patients with Type 1 Diabetes in Real-World Clinical Practice: A Noninterventional, Retrospective Chart and Database Study
- PMID: 37450196
- PMCID: PMC10363098
- DOI: 10.1007/s13300-023-01444-y
Fast-Acting Insulin Aspart in Patients with Type 1 Diabetes in Real-World Clinical Practice: A Noninterventional, Retrospective Chart and Database Study
Abstract
Introduction: This study utilized continuous glucose monitoring data to analyze the effects of switching to treatment with fast-acting insulin aspart (faster aspart) in adults with type 1 diabetes (T1D) in clinical practice.
Methods: A noninterventional database review was conducted in Sweden among adults with T1D using multiple daily injection (MDI) regimens who had switched to treatment with faster aspart as part of basal-bolus treatment. Glycemic data were retrospectively collected during the 26 weeks before switching (baseline) and up to 32 weeks after switching (follow-up) to assess changes in time in glycemic range (TIR; 70-180 mg/dL), mean sensor glucose, glycated hemoglobin (HbA1c) levels, coefficient of variation, time in hyperglycemia (level 1, > 180 to ≤ 250 mg/dL; level 2, > 250 mg/dL), and time in hypoglycemia (level 1, ≥ 54 to < 70 mg/dL; level 2, < 54 mg/dL) (ClinicalTrials.gov Identifier NCT03895515).
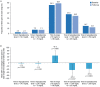
Results: Overall, 178 participants were included in the study cohort. The analysis population included 82 individuals (mean age 48.5 years) with adequate glucose sensor data. From baseline to follow-up, statistically significant improvements were reported for TIR (mean increase 3.3%-points [approximately 48 min/day]; p = 0.006) with clinically relevant improvement (≥ 5%) in 43% of participants. Statistically significant improvements from baseline were observed for mean sensor glucose levels, HbA1c levels, and time in hyperglycemia (levels 1 and 2), with no statistically significant changes in time spent in hypoglycemia.
Conclusions: Switching to faster aspart was associated with improvements in glycemic control without increasing hypoglycemia in adults with T1D using MDI in this real-world setting.
Keywords: Diabetes mellitus, type 1; Glycemic control; Insulin, short-acting; Time in range.
© 2023. The Author(s).
Conflict of interest statement
Marcus Lind has received honoraria or has been a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk, and received research grants from Eli Lilly and Novo Nordisk. Sergiu Bogdan Catrina has no conflicts of interest to disclose. Neda Rajamand Ekberg has, on behalf of her employer, received gratuities for lecturing and participating in scientific boards for Novo Nordisk. Sofia Gerward and Tariq Halasa are employees of Novo Nordisk A/S. Jarl Hellman has served on advisory boards or lectured for Abbot, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, MSD, Nordic Infucare, Novo Nordisk, Rubin Medical, and Sanofi. Detlef Hess has stock or stock options for AstraZeneca. Magnus Löndahl has received honoraria for consulting and/or lecture fees and/or unrestricted research grants from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, and Sanofi. Veronica Qvist has no conflicts of interest to disclose. Jan Bolinder has received honoraria for consulting and/or lecture fees from Abbott Diabetes Care, MannKind Corporation, Nanexa, Nordic InfuCare, Novo Nordisk, and Sanofi.
Figures


References
-
- Diabetes, Control Complications Trial/Epidemiology of Diabetes, Interventions Complications Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39(5):686–693. doi: 10.2337/dc15-1990. - DOI - PMC - PubMed
-
- Holt RIG, De Vries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2021;64(12):2609–2652. doi: 10.1007/s00125-021-05568-3. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

