Different Chronic Stress Paradigms Converge on Endogenous TDP43 Cleavage and Aggregation
- PMID: 37450246
- PMCID: PMC10533643
- DOI: 10.1007/s12035-023-03455-z
Different Chronic Stress Paradigms Converge on Endogenous TDP43 Cleavage and Aggregation
Abstract
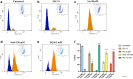
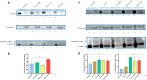
The TAR-DNA binding protein (TDP43) is a nuclear protein whose cytoplasmic inclusions are hallmarks of Amyotrophic Lateral Sclerosis (ALS). Acute stress in cells causes TDP43 mobilization to the cytoplasm and its aggregation through different routes. Although acute stress elicits a strong phenotype, is far from recapitulating the years-long aggregation process. We applied different chronic stress protocols and described TDP43 aggregation in a human neuroblastoma cell line by combining solubility assays, thioflavin-based microscopy and flow cytometry. This approach allowed us to detect, for the first time to our knowledge in vitro, the formation of 25 kDa C-terminal fragment of TDP43, a pathogenic hallmark of ALS. Our results indicate that chronic stress, compared to the more common acute stress paradigm, better recapitulates the cell biology of TDP43 proteinopathies. Moreover, we optimized a protocol for the detection of bona fide prions in living cells, suggesting that TDP43 may form amyloids as a stress response.
Keywords: Amyotrophic Lateral Sclerosis; Chronic stress; Prion; TDP43; Thioflavin.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





Similar articles
-
Interaction of RNA with a C-terminal fragment of the amyotrophic lateral sclerosis-associated TDP43 reduces cytotoxicity.Sci Rep. 2016 Jan 13;6:19230. doi: 10.1038/srep19230. Sci Rep. 2016. PMID: 26757674 Free PMC article.
-
Monoclonal full-length antibody against TAR DNA binding protein 43 reduces related proteinopathy in neurons.JCI Insight. 2020 Nov 5;5(21):e140420. doi: 10.1172/jci.insight.140420. JCI Insight. 2020. PMID: 33021970 Free PMC article.
-
An optimized InCell Western screening technique identifies hexachlorophene as a novel potent TDP43 targeting drug.J Biotechnol. 2015 Aug 10;207:34-8. doi: 10.1016/j.jbiotec.2015.04.012. Epub 2015 May 16. J Biotechnol. 2015. PMID: 25987361
-
TDP43 and RNA instability in amyotrophic lateral sclerosis.Brain Res. 2018 Aug 15;1693(Pt A):67-74. doi: 10.1016/j.brainres.2018.01.015. Epub 2018 Jan 31. Brain Res. 2018. PMID: 29395044 Free PMC article. Review.
-
Triad of TDP43 control in neurodegeneration: autoregulation, localization and aggregation.Nat Rev Neurosci. 2021 Apr;22(4):197-208. doi: 10.1038/s41583-021-00431-1. Epub 2021 Mar 2. Nat Rev Neurosci. 2021. PMID: 33654312 Review.
Cited by
-
Dysregulated FOXO1 activity drives skeletal muscle intrinsic dysfunction in amyotrophic lateral sclerosis.Acta Neuropathol. 2024 Sep 16;148(1):43. doi: 10.1007/s00401-024-02794-y. Acta Neuropathol. 2024. PMID: 39283487 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

