Monoclonal Antibody and Oral Antiviral Treatment of SARS-CoV-2 Infection in US Nursing Homes
- PMID: 37450293
- PMCID: PMC10349351
- DOI: 10.1001/jama.2023.12945
Monoclonal Antibody and Oral Antiviral Treatment of SARS-CoV-2 Infection in US Nursing Homes
Plain language summary
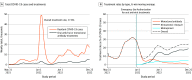
This study examines the use of COVID-19 antiviral treatments in US nursing homes and the facility characteristics associated with use of oral antivirals and monoclonal antibodies.
Conflict of interest statement
Figures
Comment in
-
Treatment of SARS-CoV-2 Infection in US Nursing Homes.JAMA. 2023 Nov 28;330(20):2021-2022. doi: 10.1001/jama.2023.20015. JAMA. 2023. PMID: 38015226 No abstract available.
References
-
- Centers for Medicare & Medicaid Services . COVID-19 nursing home data. Published 2023. Updated June 25, 2023. Accessed March 22, 2023. https://data.cms.gov/covid-19/covid-19-nursing-home-data
-
- Department of Health and Human Services . Winter playbook for nursing homes and other long-term care facilities to manage COVID-19 and protect residents, staff, and visitors. Published 2022. Accessed May 17, 2023. https://www.whitehouse.gov/wp-content/uploads/2022/12/Winter-Playbook-fo...
-
- Murphy SJ, Samson LW, Sommers BD. COVID-19 antivirals utilization: geographic and demographic patterns of treatment in 2022. Office of the Assistant Secretary for Planning and Evaluation. Published December 23, 2022. Accessed May 11, 2023. https://aspe.hhs.gov/reports/covid-19-antivirals-utilization
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

