Spending on Phased Clinical Development of Approved Drugs by the US National Institutes of Health Compared With Industry
- PMID: 37450296
- PMCID: PMC10349341
- DOI: 10.1001/jamahealthforum.2023.1921
Spending on Phased Clinical Development of Approved Drugs by the US National Institutes of Health Compared With Industry
Abstract
Importance: The launch of the Advanced Research Projects Agency for Health to advance new cures and address public concern regarding drug prices has raised questions about the roles of government and industry in drug development.
Objectives: To compare National Institutes of Health (NIH) spending on phased clinical development of approved drugs with that by industry.
Design: This cross-sectional study examined NIH funding for published research reporting the results of phased clinical trials of drugs approved between 2010 and 2019 and compared the findings with reported industry spending estimates. Data analysis was performed between May 2021 and August 2022 using PubMed data from January 1999 through October 2021 and NIH Research Portfolio Online Reporting Tools Expenditures and Results data from January 1999 through December 2020.
Exposures: Drugs approved between 2010 and 2019.
Main outcome and measures: National Institutes of Health funding for published research describing applied research on approved drugs, basic research on their biological targets, and phased clinical trials related to drugs approved between 2010 and 2019 were evaluated using Mann-Whitney U tests. All costs were inflation adjusted to 2018.
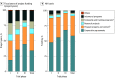
Results: National Institutes of Health funding for basic or applied research related to 386 of 387 drugs approved between 2010 and 2019 totaled $247.3 billion. Of this amount, $8.1 billion (3.3%) was related to phased clinical development. This funding contributed to 12 340 publications on phased clinical trial results involving 240 of 387 (62.0%) drugs. Average NIH spending was $33.8 million per drug, including $13.9 million per drug for phase 1, $22.2 million per drug for phase 2, and $12.9 million per drug for phase 3 trials. Spending by NIH on phased development represented 9.8% to 10.7% of estimated industry spending, including 24.6% to 25.3% of estimated phase 1, 21.4% to 23.2% of phase 2, and 3.7% to 4.3% of phase 3 costs. Considering 60 products for which estimated industry costs were publicly available, NIH spending on clinical trials was significantly lower than estimated industry spending (sum of averages, $54.9 million per drug; mean difference, $326.0 million; 95% CI, $235.6-$416.4 million; 2-tailed paired t test P < .001). More than 90% of NIH funding came through cooperative agreements or program projects and centers, while 3.3% of NIH funding came through investigator-initiated research projects.
Conclusions and relevance: In this cross-sectional study, NIH funding for phased clinical development of drugs approved between 2010 and 2019 represented a small fraction of NIH spending on pharmaceutical innovation. This spending focused primarily on early-phase clinical trials and research capacity and was significantly less than estimated industry spending on clinical development. These results may inform the efficient allocation of government funding to advance pharmaceutical innovation.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamahealthforum.2023.1309
Similar articles
-
Comparison of Research Spending on New Drug Approvals by the National Institutes of Health vs the Pharmaceutical Industry, 2010-2019.JAMA Health Forum. 2023 Apr 7;4(4):e230511. doi: 10.1001/jamahealthforum.2023.0511. JAMA Health Forum. 2023. PMID: 37115539 Free PMC article.
-
Trends in Clinical Research Including Asian American, Native Hawaiian, and Pacific Islander Participants Funded by the US National Institutes of Health, 1992 to 2018.JAMA Netw Open. 2019 Jul 3;2(7):e197432. doi: 10.1001/jamanetworkopen.2019.7432. JAMA Netw Open. 2019. PMID: 31339543 Free PMC article.
-
Rates of National Institutes of Health Funding for Surgeon-Scientists, 1995-2020.JAMA Surg. 2023 Jul 1;158(7):756-764. doi: 10.1001/jamasurg.2023.1571. JAMA Surg. 2023. PMID: 37195709 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
National institutes of health: Analysis of gender differences in anesthesiology research funding.Women Health. 2025 Feb;65(2):208-218. doi: 10.1080/03630242.2025.2460664. Epub 2025 Feb 1. Women Health. 2025. PMID: 39891539 Review.
Cited by
-
Adoption of the voluntary conflict of interest statement on PubMed.PLoS One. 2024 Oct 30;19(10):e0308782. doi: 10.1371/journal.pone.0308782. eCollection 2024. PLoS One. 2024. PMID: 39475909 Free PMC article.
-
Harnessing policy to promote inclusive medical product evidence: development of a reference standard and structured audit of clinical trial diversity policies.BMJ Med. 2024 Jul 10;3(1):e000920. doi: 10.1136/bmjmed-2024-000920. eCollection 2024. BMJ Med. 2024. PMID: 39175919 Free PMC article.
-
The strength and importance of government-funded patents for approved drugs.Nat Biotechnol. 2025 Jul;43(7):1050-1052. doi: 10.1038/s41587-025-02724-7. Nat Biotechnol. 2025. PMID: 40664847 No abstract available.
-
Lessons From Insulin: Policy Prescriptions for Affordable Diabetes and Obesity Medications.Diabetes Care. 2024 Aug 1;47(8):1246-1256. doi: 10.2337/dci23-0042. Diabetes Care. 2024. PMID: 38536964 Free PMC article. Review.
-
Internet-based enrollment of a myositis patient cohort-a national experience.Clin Rheumatol. 2024 Oct;43(10):3157-3166. doi: 10.1007/s10067-024-07091-3. Epub 2024 Aug 26. Clin Rheumatol. 2024. PMID: 39187744
References
-
- Remarks by President Biden in address to a joint session of Congress. The White House ; April 28, 2021. Accessed July 4, 2022. https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/04/29/rem...
-
- Toole AA. The impact of public basic research on industrial innovation: evidence from the pharmaceutical industry. Res Policy. 2012;41(1):1-12. doi:10.1016/j.respol.2011.06.004 - DOI

