Evaluation of Porcine Psoas Major as a Scaffold Material for Engineered Heart Tissues
- PMID: 37450340
- PMCID: PMC10618816
- DOI: 10.1089/ten.TEC.2023.0064
Evaluation of Porcine Psoas Major as a Scaffold Material for Engineered Heart Tissues
Abstract
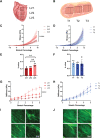
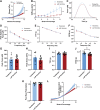
Decellularized porcine myocardium is commonly used as scaffolding for engineered heart tissues (EHTs). However, structural and mechanical heterogeneity in the myocardium complicate production of mechanically consistent tissues. In this study, we evaluate the porcine psoas major muscle (tenderloin) as an alternative scaffold material. Head-to-head comparison of decellularized tenderloin and ventricular scaffolds showed only minor differences in mean biomechanical characteristics, but tenderloin scaffolds were less variable and less dependent on the region of origin than ventricular samples. The active contractile behavior of EHTs made by seeding tenderloin versus ventricular scaffolds with human-induced pluripotent stem cell-derived cardiomyocytes was also comparable, with only minor differences observed. Collectively, the data reveal that the behavior of EHTs produced from decellularized porcine psoas muscle is almost identical to those made from porcine left ventricular myocardium, with the advantages of being more homogeneous, biomechanically consistent, and readily obtainable.
Keywords: cardiomyocyte; decellularized scaffolds; engineered heart tissue; stem cell; tissue engineering.
Conflict of interest statement
S.G.C. has equity ownership in Propria LLC, which has licensed EHT technology used in the research reported in this publication. This arrangement has been reviewed and approved by the Yale University Conflict of Interest Office. The authors declare no additional competing financial interests. J.A.K. provided consulting and collaborative studies with various pharmaceutical companies, but the content of this work is unrelated to the article.
Figures


References
-
- Ott HC, Matthiesen TS, Goh SK, et al. . Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nat Med 2008;14:213–221. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

