The impact of a combined TB/HIV intervention on the incidence of TB infection among adolescents and young adults in the HPTN 071 (PopART) trial communities in Zambia and South Africa
- PMID: 37450474
- PMCID: PMC10348566
- DOI: 10.1371/journal.pgph.0001473
The impact of a combined TB/HIV intervention on the incidence of TB infection among adolescents and young adults in the HPTN 071 (PopART) trial communities in Zambia and South Africa
Abstract
Background: HPTN071 (PopART) was a cluster randomized trial conducted in Zambian and South African (SA) communities, between 2013-2018. The PopART intervention (universal HIV-testing and treatment (UTT) combined with population-level TB symptom screening) was implemented in 14 communities. The TREATS study (2017-2021) was conducted to evaluate the impact of the PopART intervention on TB outcomes. We report on the impact of the combined TB/HIV intervention on the incidence of TB infection in a cohort of adolescents and young adults (AYA) aged 15-24 years.
Methods: A random sample of AYA was enrolled between July 2018 and July 2019 in 7 intervention vs 7 standard-of-care communities. We collected questionnaire data on risk factors for TB, and blood for measuring TB infection using QuantiFERON (QFT) Plus. AYA were seen at months 12 and 24 with all procedures repeated. Primary outcome was incidence of TB infection comparing intervention and standard-of-care communities. An incident case was defined as a participant with QFT interferon-gamma response of < 0.2 IU/ml plasma ('negative') at baseline and a QFT interferon-gamma response of > = 0.7 IU/ml ('positive') at follow up.
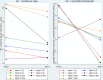
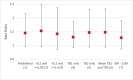
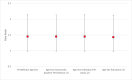
Results: We enrolled 4,648 AYA, 2,223 (47.8%) had a negative QFT-plus result at baseline, 1,902 (85.6%) had a follow up blood sample taken at 12 months or 24 months. Among the 1,902 AYA, followed for 2,987 person-years, 213 had incident TB infection giving (7.1 per 100 person-years). TB infection incidence rates were 8.7 per 100 person-years in intervention communities compared to 6.0 per 100 person-years in standard-of-care communities. There was no evidence the intervention reduced the transmission of TB (incidence-rate-ratio of 1.45, 95%CI 0.97-2.15, p = 0.063).
Conclusion: In our trial setting, we found no evidence that UTT combined with TB active case finding reduced the incidence of TB infection at population level. Our data will inform future modelling work to better understand the population level dynamics of HIV and TB.
Copyright: © 2023 Shanaube et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures

References
-
- WHO. Global tuberculosis report 2021 [Available from: https://www.who.int/publications/i/item/9789240037021
-
- Ministry of Health Zambia. Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: Final Report. Lusaka MoH, February 2019.
-
- WHO. Global Tuberculosis Report 2020 [Available from: https://www.who.int/publications/i/item/9789240013131.
Grants and funding
LinkOut - more resources
Full Text Sources
