Intensive exercise ameliorates motor and cognitive symptoms in experimental Parkinson's disease restoring striatal synaptic plasticity
- PMID: 37450585
- PMCID: PMC10348672
- DOI: 10.1126/sciadv.adh1403
Intensive exercise ameliorates motor and cognitive symptoms in experimental Parkinson's disease restoring striatal synaptic plasticity
Abstract
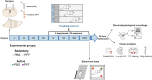
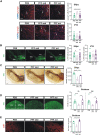
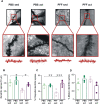
Intensive physical activity improves motor functions in patients with Parkinson's disease (PD) at early stages. However, the mechanisms underlying the beneficial effects of exercise on PD-associated neuronal alterations have not been fully clarified yet. Here, we tested the hypothesis that an intensive treadmill training program rescues alterations in striatal plasticity and early motor and cognitive deficits in rats receiving an intrastriatal injection of alpha-synuclein (α-syn) preformed fibrils. Improved motor control and visuospatial learning in active animals were associated with a recovery of dendritic spine density alterations and a lasting rescue of a physiological corticostriatal long-term potentiation (LTP). Pharmacological analyses of LTP show that modulations of N-methyl-d-aspartate receptors bearing GluN2B subunits and tropomyosin receptor kinase B, the main brain-derived neurotrophic factor receptor, are involved in these beneficial effects. We demonstrate that intensive exercise training has effects on the early plastic alterations induced by α-syn aggregates and reduces the spread of toxic α-syn species to other vulnerable brain areas.
Figures



References
-
- W. Poewe, K. Seppi, C. M. Tanner, G. M. Halliday, P. Brundin, J. Volkmann, A.-E. Schrag, A. E. Lang, Parkinson disease. Nat. Rev. Dis. Primers 3, 17013 (2017). - PubMed
-
- M. Schenkman, C. G. Moore, W. M. Kohrt, D. A. Hall, A. Delitto, C. L. Comella, D. A. Josbeno, C. L. Christiansen, B. D. Berman, B. M. Kluger, E. L. Melanson, S. Jain, J. A. Robichaud, C. Poon, D. M. Corcos, Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 75, 219–226 (2018). - PMC - PubMed
-
- M. A. Sacheli, J. L. Neva, B. Lakhani, D. K. Murray, N. Vafai, E. Shahinfard, C. English, S. McCormick, K. Dinelle, N. Neilson, J. McKenzie, M. Schulzer, D. C. McKenzie, S. Appel-Cresswell, M. J. McKeown, L. A. Boyd, V. Sossi, A. J. Stoessl, Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Mov. Disord. 34, 1891–1900 (2019). - PubMed
-
- N. M. van der Kolk, N. M. de Vries, R. P. C. Kessels, H. Joosten, A. H. Zwinderman, B. Post, B. R. Bloem, Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: A double-blind, randomised controlled trial. Lancet Neurol. 18, 998–1008 (2019). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

