Glyoxal fixation: An approach to solve immunohistochemical problem in neuroscience research
- PMID: 37450597
- PMCID: PMC10348680
- DOI: 10.1126/sciadv.adf7084
Glyoxal fixation: An approach to solve immunohistochemical problem in neuroscience research
Abstract
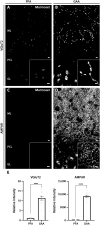
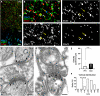
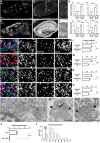
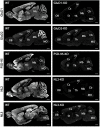
The gold-standard fixative for immunohistochemistry is 4% formaldehyde; however, it limits antibody access to target molecules that are buried within specialized neuronal components, such as ionotropic receptors at the postsynapse and voltage-gated ion channels at the axon initial segment, often requiring additional antigen-exposing techniques to detect their authentic signals. To solve this problem, we used glyoxal, a two-carbon atom di-aldehyde. We found that glyoxal fixation greatly improved antibody penetration and immunoreactivity, uncovering signals for buried molecules by conventional immunohistochemical procedures at light and electron microscopic levels. It also enhanced immunosignals of most other molecules, which are known to be detectable in formaldehyde-fixed sections. Furthermore, we unearthed several specific primary antibodies that were once judged to be unusable in formaldehyde-fixed tissues, allowing us to successfully localize so far controversial synaptic adhesion molecule Neuroligin 1. Thus, glyoxal is a highly effective fixative for immunostaining, and a side-by-side comparison of glyoxal and formaldehyde fixation is recommended for routine immunostaining in neuroscience research.
Figures




References
-
- K. Konno, M. Watanabe, Immunohistochemistry for ion channels and their interacting molecules: Tips for improving antibody accessibility, in Receptor and Ion Channel Detection in the Brain. Methods and Protocols (Neuromethods, Springer, 2016), vol. 110, pp. 171–178.
-
- A. Baude, Z. Nusser, E. Molnár, R. A. McIlhinney, P. Somogyi, High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience 69, 1031–1055 (1995). - PubMed
-
- M. Watanabe, M. Fukaya, K. Sakimura, T. Manabe, M. Mishina, Y. Inoue, Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur. J. Neurosci. 10, 478–487 (1998). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

