Mitochondria Transplantation Mitigates Damage in an In Vitro Model of Renal Tubular Injury and in an Ex Vivo Model of DCD Renal Transplantation
- PMID: 37450698
- PMCID: PMC10631499
- DOI: 10.1097/SLA.0000000000006005
Mitochondria Transplantation Mitigates Damage in an In Vitro Model of Renal Tubular Injury and in an Ex Vivo Model of DCD Renal Transplantation
Abstract
Objectives: To test whether mitochondrial transplantation (MITO) mitigates damage in 2 models of acute kidney injury (AKI).
Background: MITO is a process where exogenous isolated mitochondria are taken up by cells. As virtually any morbid clinical condition is characterized by mitochondrial distress, MITO may find a role as a treatment modality in numerous clinical scenarios including AKI.
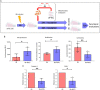
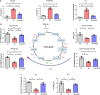
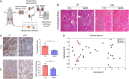
Methods: For the in vitro experiments, human proximal tubular cells were damaged and then treated with mitochondria or placebo. For the ex vivo experiments, we developed a non-survival ex vivo porcine model mimicking the donation after cardiac death renal transplantation scenario. One kidney was treated with mitochondria, although the mate organ received placebo, before being perfused at room temperature for 24 hours. Perfusate samples were collected at different time points and analyzed with Raman spectroscopy. Biopsies taken at baseline and 24 hours were analyzed with standard pathology, immunohistochemistry, and RNA sequencing analysis.
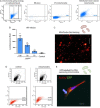
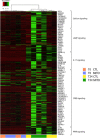
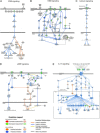
Results: In vitro, cells treated with MITO showed higher proliferative capacity and adenosine 5'-triphosphate production, preservation of physiological polarization of the organelles and lower toxicity and reactive oxygen species production. Ex vivo, kidneys treated with MITO shed fewer molecular species, indicating stability. In these kidneys, pathology showed less damage whereas RNAseq analysis showed modulation of genes and pathways most consistent with mitochondrial biogenesis and energy metabolism and downregulation of genes involved in neutrophil recruitment, including IL1A, CXCL8, and PIK3R1.
Conclusions: MITO mitigates AKI both in vitro and ex vivo.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.R. and R.S.S. are co-founders of Rametrix Technologies, on the board of which J.R. serves as President and CEO, whereas R.S.S. as Chief Technology Officer. The remaining authors report no conflicts of interest.
Figures
References
-
- Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD--fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4:1827–1831. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

