De novo missense variants in phosphatidylinositol kinase PIP5KIγ underlie a neurodevelopmental syndrome associated with altered phosphoinositide signaling
- PMID: 37451268
- PMCID: PMC10432144
- DOI: 10.1016/j.ajhg.2023.06.012
De novo missense variants in phosphatidylinositol kinase PIP5KIγ underlie a neurodevelopmental syndrome associated with altered phosphoinositide signaling
Abstract
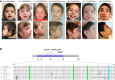
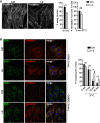
Phosphoinositides (PIs) are membrane phospholipids produced through the local activity of PI kinases and phosphatases that selectively add or remove phosphate groups from the inositol head group. PIs control membrane composition and play key roles in many cellular processes including actin dynamics, endosomal trafficking, autophagy, and nuclear functions. Mutations in phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2] phosphatases cause a broad spectrum of neurodevelopmental disorders such as Lowe and Joubert syndromes and congenital muscular dystrophy with cataracts and intellectual disability, which are thus associated with increased levels of PI(4,5)P2. Here, we describe a neurodevelopmental disorder associated with an increase in the production of PI(4,5)P2 and with PI-signaling dysfunction. We identified three de novo heterozygous missense variants in PIP5K1C, which encodes an isoform of the phosphatidylinositol 4-phosphate 5-kinase (PIP5KIγ), in nine unrelated children exhibiting intellectual disability, developmental delay, acquired microcephaly, seizures, visual abnormalities, and dysmorphic features. We provide evidence that the PIP5K1C variants result in an increase of the endosomal PI(4,5)P2 pool, giving rise to ectopic recruitment of filamentous actin at early endosomes (EEs) that in turn causes dysfunction in EE trafficking. In addition, we generated an in vivo zebrafish model that recapitulates the disorder we describe with developmental defects affecting the forebrain, including the eyes, as well as craniofacial abnormalities, further demonstrating the pathogenic effect of the PIP5K1C variants.
Keywords: PIP5K1C; de novo gain-of-function variants; developmental delay; endosomes; intellectual disability; phosphatidylinositol 4,5 bisphosphate (PI(4,5)P(2)); phosphoinositides; zebrafish.
Copyright © 2023 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- di Paolo G., de Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
-
- Downes C.P., Gray A., Lucocq J.M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. - PubMed
-
- Posor Y., Eichhorn-Grünig M., Haucke V. Phosphoinositides in endocytosis. Biochim. Biophys. Acta. 2015;1851:794–804. - PubMed
-
- Sechi A.S., Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P2 influences cytoskeletal protein activity at the plasma membrane. J. Cell Sci. 2000;113:3685–3695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

