Defining global strategies to improve outcomes in sickle cell disease: a Lancet Haematology Commission
- PMID: 37451304
- PMCID: PMC11459696
- DOI: 10.1016/S2352-3026(23)00096-0
Defining global strategies to improve outcomes in sickle cell disease: a Lancet Haematology Commission
Abstract
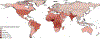
All over the world, people with sickle cell disease (an inherited condition) have premature deaths and preventable severe chronic complications, which considerably affect their quality of life, career progression, and financial status. In addition, these people are often affected by stigmatisation or structural racism, which can contribute to stress and poor mental health. Inequalities affecting people with sickle cell disease are also reflected in the distribution of the disease—mainly in sub-Saharan Africa, India, and the Caribbean—whereas interventions, clinical trials, and funding are mostly available in North America, Europe, and the Middle East. Although some of these characteristics also affect people with other genetic diseases, the fate of people with sickle cell disease seems to be particularly unfair. Simple, effective interventions to reduce the mortality and morbidity associated with sickle cell disease are available. The main obstacle preventing better outcomes in this condition, which is a neglected disease, is associated with inequalities impacting the patient populations. The aim of this Commission is to highlight the problems associated with sickle cell disease and to identify achievable goals to improve outcomes both in the short and long term.
The ambition for the management of people with sickle cell disease is that curative treatments become available to every person with the condition. Although this would have seemed unrealistic a decade ago, developments in gene therapy make this potentially achievable, albeit in the distant future. Until these curative technologies are fully developed and become widely available, health-care professionals (with the support of policy makers, funders, etc) should make sure that a minimum standard of care (including screening, prophylaxis against infection, acute medical care, safe blood transfusion, and hydroxyurea) is available to all patients.
In considering what needs to be achieved to reduce the global burden of sickle cell disease and improve the quality of life of patients, this Commission focuses on five key areas: the epidemiology of sickle cell disease (Section 1); screening and prevention (Section 2); established and emerging treatments for the management of the disease (Section 3); cellular therapies with curative potential (Section 4); and training and education needs (Section 5). As clinicians, researchers, and patients, our objective to reduce the global burden of sickle cell disease aligns with wider public health aims to reduce inequalities, improve health for all, and develop personalised treatment options. We have observed in the past few years some long-awaited momentum following the development of innovative point-of-care testing devices, new approved drugs, and emerging curative options. Reducing the burden of sickle cell disease will require substantial financial and political commitment, but it will impact the lives of millions of patients and families worldwide and the lessons learned in achieving this goal would unarguably benefit society as a whole.
Conflict of interest statement
Declaration of interests MRA has received grants or contracts with Novartis, Pfizer, Global Blood Therapeutics, and Forma Therapeutics; and participated in Data Safety Monitoring Boards or Advisory Boards for Vertex, GBT, Forma, Novartis, and Aggios. BA has participated in Data Safety Monitoring Boards or Advisory Boards for Agios, Aruvant, bluebird bio, CRISPR, Accordant (CVS), Emmaus, Forma Therapeutics, Fulcrum, Global Blood Therapeutics, Hemanext, Novartis, NovoNordisk, and Vertex. RF has received grants from the European Horizon 2020 and Horizon Europe schemes and a contract with Global Blood Therapeutics; received consulting fees from Novartis and Global Blood Therapeutics; received honoraria for a Physicians’ Education Resource and from Global Blood Therapeutics; participated in Data Safety Monitoring Boards or Advisory Boards for Vertex, Addmedica and Novonordisk; and a leadership or fiduciary role as a member of the Steering Committee and Coordinator of the Red Cell Disorder Working Group of the Italian Association of Pediatric Hematology Oncology. NC has received a research grant for Novartis Precision Medicine. MdM has received honoraria for presentations by Addmedica and Novartis and support from Addmedica for attending a conference; and participated in a Data Safety Monitoring Board or Advisory Board for Addmedica, Vertex, and Novartis. JE has received grants from the European Horizon 2020 scheme and participated in the Data Safety Monitoring Board or Advisory Board of the Fondation Pierre Fabre. EE has received consulting fees from bluebird bio. ALG has an honorary advisory role on the Australian Sickle Cell Advisory Board. IMI has received a grant from the American Society of Hematology; consulting fees from Agios Pharmaceuticals; and honoraria from the American Society of Hematology. LCJ received royalties for a book chapter. AAK has received support from the Health resources and Services Administration. MLP has received consulting fees from the American College of Medical Genetics and Genomics; and participated in a Data Safety Monitoring Board or Advisory Board for the American College of Medical Genetics and Genomics and the American Academy of Pediatrics. ON has received travel support from the American Society of Hematology to attend a conference. DCR has received consulting fees from Vertex, Forma, Agios, and Vifor; received honoraria from Vertex; and participated in a Data Safety Monitoring Board or Advisory Board for TauRx and Mitsubishi. AS has received a scholar award from the American Society of Hematology, an advancing cures research award from the Doris Duke Charitable Foundation, and a Working group award from the Center for ELSI Resources and Analysis; consulting fees from Spotlight Therapeutics, Medexus, Vertex, Editas Medicine, and Sangamo Therapeutics; has received honoraria from Vindico Medical Education; and has a leadership or fiduciary role in the American Society for Transplantation and Cellular Therapy Content Committee, the Scientific Executive Committee, Sickle Cell Transplant Advocacy & Research Alliance, and the Pediatric Transplantation and Cellular Therapy Consortium Supportive Care Committee; and has financial interests with CRISPR Therapeutics, Vertex Pharmaceuticals, Novartis Pharmaceuticals, Magenta Therapeutics, and Beam Therapeutics. AAT has received consulting fees from GlaxoSmithKline and Global Blood Therapeutics; and honoraria from Novartis. REW has received consulting fees from Nova Laboratories; participated in a Data Safety Monitoring Board for Novartis and Editas; and received donations and support for clinical trials from Bristol Myers Squib, Addmedica, and Hemex Health. DJW has received a grant or contract from the US National Institutes for Health; received payment for expert testimony from Fredslough Law Firm; participated in a Data Safety Monitoring Board or Advisory board for Northwestern University; and is the founder and chairman of eNursing.
Figures
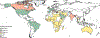
Comment in
-
Social determinants of health: the next frontier for improving care and outcomes in sickle cell disease.Lancet Haematol. 2023 Aug;10(8):e571-e573. doi: 10.1016/S2352-3026(23)00185-0. Epub 2023 Jul 11. Lancet Haematol. 2023. PMID: 37451302 No abstract available.
-
Sickle cell disease strategies and priorities.Lancet Haematol. 2023 Oct;10(10):e793-e794. doi: 10.1016/S2352-3026(23)00266-1. Lancet Haematol. 2023. PMID: 37793765 No abstract available.
-
Sickle cell disease strategies and priorities.Lancet Haematol. 2023 Oct;10(10):e794-e795. doi: 10.1016/S2352-3026(23)00267-3. Lancet Haematol. 2023. PMID: 37793766 No abstract available.
References
-
- GBD 2021 Sickle Cell Disease Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol 2023; published online June 15. 10.1016/S2352-3026(23)00118-7. - DOI - PMC - PubMed
-
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers 2018; 4: 18010. - PubMed
-
- de la Fuente J, Gluckman E, Makani J, et al. The role of haematopoietic stem cell transplantation for sickle cell disease in the era of targeted disease-modifying therapies and gene editing. Lancet Haematol 2020; 7: e902–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

