COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn
- PMID: 37451878
- PMCID: PMC10261713
- DOI: 10.1016/j.vaccine.2023.06.032
COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn
Abstract
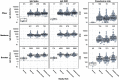
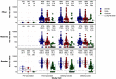
The immune response to COVID-19 booster vaccinations during pregnancy for mothers and their newborns and the functional response of vaccine-induced antibodies against Omicron variants are not well characterized. We conducted a prospective, multicenter cohort study of participants vaccinated during pregnancy with primary or booster mRNA COVID-19 vaccines from July 2021 to January 2022 at 9 academic sites. We determined SARS-CoV-2 binding and live virus and pseudovirus neutralizing antibody (nAb) titers pre- and post-vaccination, and at delivery for both maternal and infant participants. Immune responses to ancestral and Omicron BA.1 SARS-CoV-2 strains were compared between primary and booster vaccine recipients in maternal sera at delivery and in cord blood, after adjusting for days since last vaccination. A total of 240 participants received either Pfizer or Moderna mRNA vaccine during pregnancy (primary 2-dose series: 167; booster dose: 73). Booster vaccination resulted in significantly higher binding and nAb titers, including to the Omicron BA.1 variant, in maternal serum at delivery and in cord blood compared to a primary 2-dose series (range 0.44-0.88 log10 higher, p < 0.0001 for all comparisons). Live virus nAb to Omicron BA.1 were present at delivery in 9 % (GMT ID50 12.7) of Pfizer and 22 % (GMT ID50 14.7) of Moderna primary series recipients, and in 73 % (GMT ID50 60.2) of mRNA boosted participants (p < 0.0001), although titers were significantly lower than to the D614G strain. Transplacental antibody transfer was efficient for all regimens with median transfer ratio range: 1.55-1.77 for IgG, 1.00-1.78 for live virus nAb and 1.79-2.36 for pseudovirus nAb. COVID-19 mRNA vaccination during pregnancy elicited robust immune responses in mothers and efficient transplacental antibody transfer to the newborn. A booster dose during pregnancy significantly increased maternal and cord blood binding and neutralizing antibody levels, including against Omicron BA.1. Findings support the use of a booster dose of COVID-19 vaccine during pregnancy.
Keywords: Booster vaccination; COVID-19; Neutralizing antibodies; Newborn; Pregnancy; SARS-CoV-2; Transplacental antibody.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: F.M.M. is an investigator of pediatric studies of COVID-19 vaccines for Pfizer and for a pediatric remdesivir study conducted by Gilead Sciences, Inc; serves as investigator on projects supported by an NIH contract for a Vaccine Treatment and Evaluation Unit (VTEU), serves as member of the Data Safety monitoring Board (DSMB) for clinical trials conducted by Pfizer, Moderna, Meissa Vaccines, Virometix, and the NIH; and is a member of the American Academy of Pediatrics Section of Infectious Diseases (SOID), the Immunization Expert Group of the American College of Obstetrics and Gynecology (ACOG), and was co-Chair of the COVAX-CEPI Maternal Immunization Working Group. K.M.N. is a member of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization, serves as co-investigator on an NIH contract for a Vaccine Treatment and Evaluation Unit (VTEU), serves as Co-Chair of the NIH COVID Prevention Network (CoVPN), and served as an investigator for Phase I/II Pfizer COVID-19 vaccine grant, with a grant to the institution, but no salary support. M.J.M. conducts laboratory research and clinical trials with contract funding for vaccines or MABs vs SARS-CoV-2 with Lilly, Pfizer, and Sanofi and receives personal fees for Scientific Advisory Board service from Merck, Meissa Vaccines, Inc. and Pfizer. M.S.S. served as an advisor for Moderna (ended December 2021) and is currently serving as an advisor for Ocugen, Inc. B.A.R. currently holds a position on a DSMB for clinical trials at Gilead Sciences, Inc. R.C.B. at Cincinnati Children’s Hospital receives research grant support for clinical trials from PATH, Astra Zeneca and Pfizer on which she serves as co-investigator. B.B. owns shares in HDT Bio Corp. J.S.G. receives research funds from NIH for Moderna KidCOVE study. R.M.N. is a paid advisor to Gilead and an investigator on NIH-funded trials of Moderna, Pfizer and Janssen vaccines. J.R-K is a medical speaker for Abbott Nutrition with the UIC team. A.R.F. holds research grants from Pfizer, Janssen, Merck, Cyanvac, Biofire Diagnostics and serves on the DSMB for Novavax. N.R. receives funds to conduct industry trials from Pfizer, Merck, and Sanofi-Pasteur and serves as a safety consultant for EMMES and ICON. R.W.F. Jr., MD has received funds to conduct industry trials from Pfizer, Moderna and Astra Zeneca, serves on advisory boards for Merck, Sanofi-Pasteur, Johnson and Johnson and Seqirus and serves on an ICON-sponsored DSMB for a C difficile study.
Figures
Update of
-
COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn (DMID 21-0004).medRxiv [Preprint]. 2022 Jun 13:2022.06.13.22276354. doi: 10.1101/2022.06.13.22276354. medRxiv. 2022. Update in: Vaccine. 2023 Aug 14;41(36):5296-5303. doi: 10.1016/j.vaccine.2023.06.032. PMID: 35734087 Free PMC article. Updated. Preprint.
References
-
- Zambrano L.D., Ellington S., Strid P., et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. - DOI - PMC - PubMed
-
- Vousden N, Ramakrishnan R, Bunch K, et al. Management and implications of severe COVID-19 in pregnancy in the UK: data from the UK Obstetric Surveillance System national cohort. Acta Obstet Gynecol Scand. 2022 Apr;101(4):461-470. doi: 10.1111/aogs.14329. Epub 2022 Feb 25. PMID: 35213734; PMCID: PMC9111211. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

