Novelty in improvement of CAR T cell-based immunotherapy with the aid of CRISPR system
- PMID: 37451978
- PMCID: PMC10935483
- DOI: 10.1016/j.htct.2023.05.009
Novelty in improvement of CAR T cell-based immunotherapy with the aid of CRISPR system
Abstract
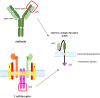
Introduction: Chimeric Antigen Receptor (CAR) T cells have tremendous potentials for cancer treatment; however, various challenges impede their universal use. These restrictions include the poor function of T cells in tumor microenvironments, the shortage of tumor-specific antigens and, finally, the high cost and time-consuming process, as well as the poor scalability of the method. Creative gene-editing tools have addressed each of these limitations and introduced next generation products for cell therapy. The clustered regularly interspaced short palindromic repeats-associated endonuclease 9 (CRISPR/Cas9) system has triggered a revolution in biology fields, as it has a great capacity for genetic manipulation.
Method: In this review, we considered the latest development of CRISPR/Cas9 methods for the chimeric antigen receptor T cell (CAR T)-based immunotherapy.
Results: The ability of the CRISPR/Cas9 system to generate the universal CAR T cells and also potent T cells that are persistent against exhaustion and inhibition was explored.
Conclusion: We explained CRISPR delivery methods, as well as addressing safety concerns related to the use of the CRISPR/Cas9 system and their potential solutions.
Keywords: Adoptive immunotherapy; CRISPR/Cas9; Cancer treatment; Chimeric antigen receptor; Gene therapy.
Copyright © 2023. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

