Epidemic thresholds and human mobility
- PMID: 37452118
- PMCID: PMC10349094
- DOI: 10.1038/s41598-023-38395-0
Epidemic thresholds and human mobility
Abstract
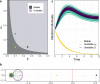
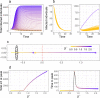
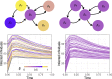
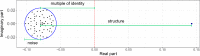
A comprehensive view of disease epidemics demands a deep understanding of the complex interplay between human behaviour and infectious diseases. Here, we propose a flexible modelling framework that brings conclusions about the influence of human mobility and disease transmission on early epidemic growth, with applicability in outbreak preparedness. We use random matrix theory to compute an epidemic threshold, equivalent to the basic reproduction number [Formula: see text], for a SIR metapopulation model. The model includes both systematic and random features of human mobility. Variations in disease transmission rates, mobility modes (i.e. commuting and migration), and connectivity strengths determine the threshold value and whether or not a disease may potentially establish in the population, as well as the local incidence distribution.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pastore y Piontti A, Perra N, Rossi L, Samay N, Vespignani A. Charting the Next Pandemic: Modeling Infectious Disease Spreading in the Data Science Age. Springer; 2019.
-
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; 1992.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

